Mechanistic Investigation on an NHC-Catalyzed [3 + 3] Annulation of 2-Bromoenals with β-Oxodithioester: Mechanism, Origins of Stereoselectivity
IF 4
2区 化学
Q2 CHEMISTRY, APPLIED
引用次数: 0
Abstract
An increasing number of studies have demonstrated the cooperative use of chiral N-heterocyclic carbenes (NHCs) and Lewis acid to enhance catalytic efficiency in stereoselective annulation reactions. Herein, molecular-level insights into the NHC-catalyzed [3 + 3] annulation of 2-bromoenals with β-oxodithioester are elucidated through density functional theory calculations. The reaction proceeds via multiple key steps: formation of the Breslow intermediate, CBr bond cleavage, α-protonation generating α,β-unsaturated acylazolium, stereoselective Si-face addition between the acylazolium and LiCl-coordinated β-oxodithioester, intramolecular proton transfer, and catalyst regeneration. The stereoselectivity-determining CC bond formation step exhibits enhanced facial control through cooperative effects. Noncovalent interaction (NCI) and atoms-in-molecules analyses reveal that hydrogen-bond networks, LP···π, and O···S interactions preferentially stabilize the RS-configured isomer. Notably, LiCl plays dual roles: (1) it amplifies stereocontrol by facilitating multiple NCIs during the CC bond formation, and (2) reduces transition-state polarity through solvent–solute interactions, thereby lowering the activation barrier. The work deepens the understanding of how Lewis acid additive modulate both electronic and steric landscapes in NHC catalysis, offering valuable insights for stereocontrolled annulation reactions.
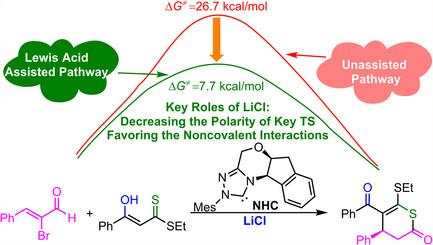
nhc催化2-溴烯醛与β-氧二硫酯[3 + 3]环化的机理研究:立体选择性的机理和来源
越来越多的研究表明,手性n -杂环碳烯(NHCs)和路易斯酸的协同使用可以提高立体选择性环化反应的催化效率。本文通过密度泛函理论计算阐明了nhc催化的2-溴烯醛与β-氧二硫酯的[3 + 3]环化反应的分子水平。该反应通过多个关键步骤进行:Breslow中间体的形成、C - Br键的裂解、α-质子化生成α、β-不饱和酰基唑、酰基唑与licl配位的β-氧二硫酯之间的立体选择性硅面加成、分子内质子转移和催化剂再生。立体选择性决定C - C键形成步骤通过协同效应表现出增强的面部控制。非共价相互作用(NCI)和原子-分子分析表明,氢键网络、LP··π和O··S相互作用优先稳定rs构型异构体。值得注意的是,LiCl具有双重作用:(1)通过促进C -羟基- C键形成过程中的多个NCIs来增强立体控制;(2)通过溶剂-溶质相互作用降低过渡态极性,从而降低激活势垒。这项工作加深了对路易斯酸添加剂如何调节NHC催化中的电子和立体景观的理解,为立体控制环化反应提供了有价值的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Advanced Synthesis & Catalysis
化学-应用化学
CiteScore
9.40
自引率
7.40%
发文量
447
审稿时长
1.8 months
期刊介绍:
Advanced Synthesis & Catalysis (ASC) is the leading primary journal in organic, organometallic, and applied chemistry.
The high impact of ASC can be attributed to the unique focus of the journal, which publishes exciting new results from academic and industrial labs on efficient, practical, and environmentally friendly organic synthesis. While homogeneous, heterogeneous, organic, and enzyme catalysis are key technologies to achieve green synthesis, significant contributions to the same goal by synthesis design, reaction techniques, flow chemistry, and continuous processing, multiphase catalysis, green solvents, catalyst immobilization, and recycling, separation science, and process development are also featured in ASC. The Aims and Scope can be found in the Notice to Authors or on the first page of the table of contents in every issue.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


