Lipid monolayer composition and production efficiency in DSPC/PEG40St microbubbles for ultrasound applications
IF 5.2
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
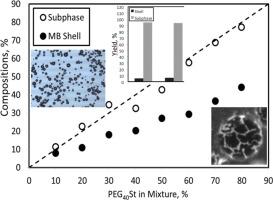
Lipid-coated microbubbles are widely used as ultrasound contrast agents (UCAs) and are being developed as carriers for drug and gene delivery. These microbubbles typically consist of an inert gas core and a stabilizing monolayer shell of phospholipid and a PEGylated emulsifier. In practice, a 9:1 M ratio of DSPC (a saturated phospholipid) to PEG-40-stearate (PEG40St) is conventionally used, under a long-standing assumption that the final composition of the microbubble shell is identical to the initial mixture composition. In this study, we tested that assumption over a wide range of DSPC/PEG40St ratios. Using sonication-based fabrication, we prepared microbubble suspensions with PEG40St fractions from 10 % up to 90 %. We then quantified the shell composition by proton nuclear magnetic resonance (1H NMR) and measured microbubble yield. Contrary to expectation, the PEG40St content in the bubble shells lower than PEG40St added, indicating selective exclusion or “squeezing out” of PEG40St during formation. Only about 4–6 % of the total lipid mixture ended up in the bubble shells and the rest remained as excess in the sub-phase. Thus, 94–96 % of the costly lipid/emulsifier was wasted in the production process. These results overturn the conventional assumption and highlight a critical inefficiency such that substantial amounts of lipid and PEG40St were lost during production, and the bubble yields were low. The findings have important implications for microbubble manufacturing, suggesting that alternative formulations or other production methods are needed to improve efficiency, and thus reduce costs.
超声应用dsc /PEG40St微泡的脂质单层组成和生产效率。
脂质包被微泡被广泛用作超声造影剂(UCAs),并正在开发作为药物和基因传递的载体。这些微泡通常由惰性气体核和磷脂的稳定单层壳和聚乙二醇化乳化剂组成。在实践中,通常使用dsc(饱和磷脂)与peg -40-硬脂酸酯(PEG40St)的比例为9:1 M,长期假设微泡壳的最终组成与初始混合物组成相同。在本研究中,我们在大范围的dsc /PEG40St比率上测试了这一假设。利用基于超声的制备,我们制备了PEG40St分数从10 %到90 %的微泡悬浮液。然后用质子核磁共振(1H NMR)定量了壳层组成,并测量了微泡产率。与预期相反,气泡壳中的PEG40St含量低于添加的PEG40St,表明在形成过程中PEG40St被选择性排除或“挤出”。只有约4- 6% %的总脂质混合物最终进入气泡壳,其余部分在亚相中剩余。因此,在生产过程中浪费了94-96 %昂贵的脂质/乳化剂。这些结果推翻了传统的假设,突出了生产过程中大量脂质和PEG40St的损失,气泡收率很低。这一发现对微泡制造具有重要意义,表明需要替代配方或其他生产方法来提高效率,从而降低成本。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
10.70
自引率
8.60%
发文量
951
审稿时长
72 days
期刊介绍:
The International Journal of Pharmaceutics is the third most cited journal in the "Pharmacy & Pharmacology" category out of 366 journals, being the true home for pharmaceutical scientists concerned with the physical, chemical and biological properties of devices and delivery systems for drugs, vaccines and biologicals, including their design, manufacture and evaluation. This includes evaluation of the properties of drugs, excipients such as surfactants and polymers and novel materials. The journal has special sections on pharmaceutical nanotechnology and personalized medicines, and publishes research papers, reviews, commentaries and letters to the editor as well as special issues.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


