Randomized Trial of Dapagliflozin in Patients With Nondiabetic Stage 4 CKD
IF 5.7
2区 医学
Q1 UROLOGY & NEPHROLOGY
引用次数: 0
Abstract
Introduction
Sodium-glucose cotransporter-2 (SGLT2) inhibitors are nephroprotective in patients with chronic kidney disease (CKD) and mild-to-moderate renal insufficiency.
Methods
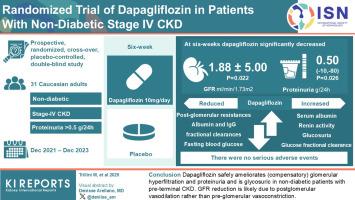
This prospective, randomized, cross-over, placebo-controlled, double-blind study compared the effects of 6-week dapagliflozin (10 mg/d) with placebo treatment in 31 consenting nondiabetic Caucasian adults with stage 4 CKD and proteinuria > 0.5 g/24 h. Participants were identified at the Nephrology Unit of Papa Giovanni XXIII Hospital and treated at Mario Negri Institute (Bergamo, Italy) between December 2021 and December 2023. Normalized glomerular filtration rate (GFR) (using iohexol plasma clearance) and 24-hour proteinuria (median of 3 urinary measurements) were co–primary outcomes. Analyses were by modified intention-to-treat.
Results
At 6 weeks, dapagliflozin significantly decreased GFR by 1.88 ± 5.00 ml/min per 1.73 m2 (P = 0.022) and proteinuria by 0.50 (−0.10 to 0.80) g/24 h (P = 0.026) versus placebo. The dapagliflozin-induced GFR (P < 0.001) and proteinuria (P = 0.003) reduction was already significant at 1 week. At 6 weeks, dapagliflozin reduced absolute GFR (P = 0.026), the CKD-Epidemiology Collaboration (CKD-Epi) equation–based estimated GFR (eGFR) (P = 0.003), the Modification of Diet in Renal Disease (MDRD) equation–based eGFR (P = 0.002), 24-hour albuminuria (P = 0.001), total protein (P = 0.057) and albumin (P = 0.009) fractional clearances, and fasting blood glucose (P < 0.001); and increased serum albumin (P = 0.001), renin activity (P = 0.020), glucosuria (P < 0.001), and glucose fractional clearance (P < 0.001) versus placebo. All changes reversed completely after treatment withdrawal. GFR changes correlated inversely with changes in renal plasma flow (RPF) (P = 0.010) and positively with changes in postglomerular resistance (P < 0.001) but did not correlate with changes in preglomerular resistance. There were no serious adverse events.
Conclusion
Dapagliflozin safely ameliorates (compensatory) glomerular hyperfiltration and proteinuria and is glycosuric in nondiabetic patients with preterminal CKD. GFR reduction is likely because of postglomerular vasodilation rather than preglomerular vasoconstriction.
达格列净在非糖尿病4期CKD患者中的随机试验
钠-葡萄糖共转运蛋白-2 (SGLT2)抑制剂对慢性肾病(CKD)和轻中度肾功能不全患者具有肾保护作用。该前瞻性、随机、交叉、安慰剂对照、双盲研究比较了31名同意的非糖尿病4期CKD和蛋白尿(0.5 g/24小时)白种成人患者6周达格列净(10mg /d)和安慰剂治疗的效果。参与者于2021年12月至2023年12月在Papa Giovanni XXIII医院肾病科确定,并在Mario Negri研究所(意大利贝加莫)接受治疗。正常的肾小球滤过率(GFR)(使用碘己醇血浆清除率)和24小时蛋白尿(3次尿测量的中位数)是共同的主要结局。采用改良意向治疗法进行分析。结果6周时,与安慰剂相比,达格列净显著降低GFR(1.88±5.00 ml/min / 1.73 m2) (P = 0.022)和蛋白尿(0.50 (- 0.10 ~ 0.80)g/24 h) (P = 0.026)。达格列净诱导的GFR (P < 0.001)和蛋白尿(P = 0.003)降低在1周时已经很显著。6周时,达格列净降低了绝对GFR (P = 0.026)、基于ckd流行病学合作(CKD-Epi)方程的估计GFR (eGFR) (P = 0.003)、基于肾脏疾病饮食调整(MDRD)方程的eGFR (P = 0.002)、24小时蛋白尿(P = 0.001)、总蛋白(P = 0.057)和白蛋白(P = 0.009)分数清除率和空腹血糖(P < 0.001);与安慰剂相比,血清白蛋白(P = 0.001)、肾素活性(P = 0.020)、血糖(P < 0.001)和葡萄糖分数清除率(P < 0.001)增加。停药后所有变化完全逆转。GFR变化与肾血浆流量(RPF)变化呈负相关(P = 0.010),与肾小球后阻力变化呈正相关(P < 0.001),但与肾小球前阻力变化无相关性。无严重不良事件发生。结论达格列净可安全改善非糖尿病晚期CKD患者(代偿性)肾小球高滤过、蛋白尿和糖尿。GFR降低可能是因为肾小球后血管扩张而不是肾小球前血管收缩。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Kidney International Reports
Medicine-Nephrology
CiteScore
7.70
自引率
3.30%
发文量
1578
审稿时长
8 weeks
期刊介绍:
Kidney International Reports, an official journal of the International Society of Nephrology, is a peer-reviewed, open access journal devoted to the publication of leading research and developments related to kidney disease. With the primary aim of contributing to improved care of patients with kidney disease, the journal will publish original clinical and select translational articles and educational content related to the pathogenesis, evaluation and management of acute and chronic kidney disease, end stage renal disease (including transplantation), acid-base, fluid and electrolyte disturbances and hypertension. Of particular interest are submissions related to clinical trials, epidemiology, systematic reviews (including meta-analyses) and outcomes research. The journal will also provide a platform for wider dissemination of national and regional guidelines as well as consensus meeting reports.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


