Fixed-bed breakthrough curve modeling for effective samarium adsorption on radiated-grafted-vinyl acetate-polypropylene fibrous
IF 9
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
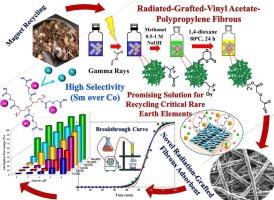
The research involved exposing polypropylene polymer fibers to gamma-ray irradiation in order to attach vinyl acetate monomers to the fibers. Next, the grafted polymer was hydrolyzed and then placed in a solution containing diglycolic anhydride and 1,4-dioxane to attach the ligand, resulting in the creation of a modified polymer adsorbent with effective samarium ion adsorption capabilities. Various factors influencing samarium adsorption were studied, such as contact time, pH levels, temperature, adsorbent weight, and samarium ion concentration. The structure of the adsorbent was analyzed using FTIR and SEM techniques, revealing a significant grafting percentage with a well-structured material. Both equilibrium isotherms and kinetics were examined, with the results aligning with the Langmuir and pseudo second order models. Research involving the adsorption process of rare earths, copper, zinc, and cobalt indicated that samarium and other rare earth ions are specifically adsorbed at a pH of 3, while cobalt ions do not exhibit significant adsorption. This finding suggests that this process could be a viable option for treating leach solutions resulting from the recovery of samarium and cobalt magnets. The research conducted a comparison of five distinct models for breakthrough curves to simulate the dynamic adsorption process. Modeling the breakthrough curve characteristics aided in comprehending the dynamic behavior of the selected adsorbent during the adsorption of samarium.
辐射接枝醋酸乙烯-聚丙烯纤维有效吸附钐的固定床突破曲线模拟
这项研究包括将聚丙烯聚合物纤维暴露在伽马射线照射下,以便将醋酸乙烯单体附着在纤维上。然后,将接枝聚合物水解,然后将其置于含有二乙醇酸酐和1,4-二恶烷的溶液中,使其与配体结合,从而产生具有有效钐离子吸附能力的改性聚合物吸附剂。研究了接触时间、pH值、温度、吸附剂质量、钐离子浓度等因素对钐吸附的影响。利用FTIR和SEM技术分析了吸附剂的结构,发现在结构良好的材料上接枝率很高。研究了平衡等温线和动力学,结果与Langmuir和伪二阶模型一致。对稀土、铜、锌、钴的吸附过程的研究表明,在pH为3时,钐等稀土离子被特异性吸附,而钴离子没有明显的吸附。这一发现表明,该工艺可能是处理钐和钴磁体回收产生的浸出液的可行选择。研究对五种不同的突破曲线模型进行了比较,以模拟动态吸附过程。对突破曲线特征进行建模有助于理解所选吸附剂在吸附钐过程中的动态行为。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Separation and Purification Technology
工程技术-工程:化工
CiteScore
14.00
自引率
12.80%
发文量
2347
审稿时长
43 days
期刊介绍:
Separation and Purification Technology is a premier journal committed to sharing innovative methods for separation and purification in chemical and environmental engineering, encompassing both homogeneous solutions and heterogeneous mixtures. Our scope includes the separation and/or purification of liquids, vapors, and gases, as well as carbon capture and separation techniques. However, it's important to note that methods solely intended for analytical purposes are not within the scope of the journal. Additionally, disciplines such as soil science, polymer science, and metallurgy fall outside the purview of Separation and Purification Technology. Join us in advancing the field of separation and purification methods for sustainable solutions in chemical and environmental engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


