Efficient green synthesis of ammonia: from mechanistic understanding to reactor design for potential production
IF 39
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
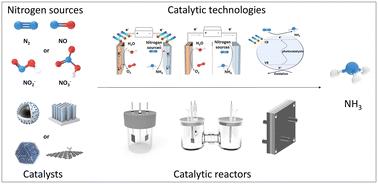
Ammonia (NH3), one of the world's most vital chemicals and energy carriers, has attracted wide attention. Currently, NH3 is mainly produced using the traditional, energy-intensive Haber–Bosch (H–B) technology, which has a large impact on the environment. Therefore, developing a low-cost, high-efficiency, and eco-friendly way to produce NH3 is highly desirable. Photo-, electro-, photoelectro-, and alkali–metal-mediated catalytic reactions powered by renewable and clean energy under ambient conditions offer alternatives to the H–B process and have recently gained significant interest. However, efficient nitrogen reduction is a key requirement, limiting the selectivity and activity for the green synthesis of NH3 because the N2 activation process in a green catalytic system is difficult to complete due to its thermodynamic instability and chemical inertness. Compared to the reduction of N2, the catalytic reduction of some soluble and harmful high-valent sources (e.g., NO, NO2−, and NO3−) is considered an effective method for increasing NH3 synthesis efficiency. This review article focuses on the important features of the green catalytic conversion of multiple nitrogen resources into NH3 by summarizing the fundamental mechanistic understanding, catalytic descriptors, and current advances, along with the various catalysts used for these conversion strategies and their structure–activity relationships. Meanwhile, opportunities and prospects for reactor design and construction for potential NH3 production at high current densities are also discussed, focusing on achieving a high yield rate, Faraday efficiency, and energy efficiency. This will provide valuable guidance for constructing catalysts and optimizing reaction systems that can meet the needs of practical applications.
高效的绿色合成氨:从机理理解到潜在生产的反应器设计
氨(NH3)作为世界上最重要的化学物质和能源载体之一,引起了人们的广泛关注。目前,NH3的生产主要采用传统的、高能耗的Haber-Bosch (H-B)工艺,该工艺对环境影响较大。因此,开发一种低成本、高效率、环保的方法来生产NH3是非常必要的。在环境条件下,由可再生能源和清洁能源驱动的光、电、光电和碱金属介导的催化反应提供了H-B过程的替代品,最近引起了人们的极大兴趣。然而,高效的氮还原是一个关键要求,这限制了绿色合成NH3的选择性和活性,因为绿色催化体系中的N2活化过程由于其热力学不稳定性和化学惰性而难以完成。与N2的还原相比,催化还原一些可溶和有害的高价源(如NO、NO2−和NO3−)被认为是提高NH3合成效率的有效方法。本文综述了多种氮资源绿色催化转化为NH3的基本机理、催化描述符、目前研究进展,以及用于这些转化策略的各种催化剂及其构效关系,重点介绍了多种氮资源绿色催化转化为NH3的重要特征。同时,还讨论了在高电流密度下潜在NH3生产的反应堆设计和建造的机会和前景,重点是实现高收率、法拉第效率和能源效率。这将为构建满足实际应用需要的催化剂和优化反应体系提供有价值的指导。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Society Reviews
化学-化学综合
CiteScore
80.80
自引率
1.10%
发文量
345
审稿时长
6.0 months
期刊介绍:
Chemical Society Reviews is published by: Royal Society of Chemistry.
Focus: Review articles on topics of current interest in chemistry;
Predecessors: Quarterly Reviews, Chemical Society (1947–1971);
Current title: Since 1971;
Impact factor: 60.615 (2021);
Themed issues: Occasional themed issues on new and emerging areas of research in the chemical sciences
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


