High efficiency adsorption of Cd(II) by KHCO3-activated Fe/Mn co-modified peanut shell biochar: adsorption performance, mechanisms, and life cycle assessment
IF 4.3
2区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
Cadmium (Cd) is a ubiquitous environmental pollutant in aqueous solution worldwide, posing serious risk to human health and ecosystem stability. Adsorption using efficient adsorbents is considered the most promising method to remove Cd(II). To achieve efficient removal of Cd(II), a low-cost, environmental friendly KHCO3-activated Fe/Mn co-modified peanut shell biochar (K-FM-BC) was synthesized by one-pot method, its Cd(II) adsorption performance and mechanisms and potential environmental impact are elucidated in detail via batch adsorption, multi-spectroscopic characterization, density functional theory calculation (DFT), and Life cycle assessment (LCA). The results showed that K-FM-BC was covered by FeO and MnO particles with uneven and rough surface, its adsorption of Cd(II) was fitted to the Langmuir and pseudo-second-order kinetic models, with the theoretical maximum adsorption capacity (657.89 mg/g) much higher than many previous studies. Fourier-transform infrared spectrometer, X-ray photoelectron spectroscopy, and Zeta potential analysis showed that Cd(II) adsorption mainly involve the mechanisms of complexation, co-precipitation, and electrostatic attraction. DFT analysis showed that oxygen-containing functional groups play a decisive role in Cd(II) adsorption by K-FM-BC. Additionally, Fe- and Mn- based active sites exhibit stronger binding capacity for Cd(II), with the adsorption energies of −64.77 and −69.18 kcal/mol, respectively. LCA analysis revealed that K-FM-BC production generates 6.20 kg CO2 eq emissions, with KHCO3 as the primary contributor of the environmental impact (84 %). Cost-benefit analysis revealed that K-FM-BC demonstrates excellent cost-effectiveness among various adsorbents (96.28 g Cd/$). These results indicate that K-FM-BC has great potential in remediating Cd(II)-contaminated wastewater.
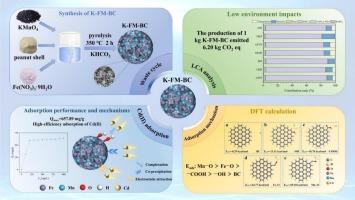
khco3活化Fe/Mn共改性花生壳生物炭对Cd(II)的高效吸附:吸附性能、机理及生命周期评价
镉(Cd)是一种普遍存在于水溶液中的环境污染物,对人类健康和生态系统稳定构成严重威胁。高效吸附剂的吸附被认为是最有前途的去除Cd(II)的方法。为达到高效脱除Cd(II)的目的,采用一锅法合成了低成本、环境友好的khco3活化Fe/Mn共改性花生壳生物炭(K-FM-BC),并通过批吸附、多光谱表征、密度泛函数理论计算(DFT)和生命周期评价(LCA)对其Cd(II)的吸附性能、机理和潜在环境影响进行了详细研究。结果表明,K-FM-BC表面覆盖有表面凹凸不平的FeO和MnO颗粒,其对Cd(II)的吸附符合Langmuir和拟二阶动力学模型,理论最大吸附量为657.89 mg/g,远高于前人研究。傅里叶变换红外光谱、x射线光电子能谱和Zeta电位分析表明,Cd(II)的吸附机制主要包括络合、共沉淀和静电吸引。DFT分析表明,含氧官能团对K-FM-BC吸附Cd(II)起决定性作用。此外,Fe基和Mn基活性位点对Cd(II)的吸附能力较强,吸附能分别为- 64.77和- 69.18 kcal/mol。LCA分析显示,K-FM-BC生产产生6.20 kg CO2当量排放,其中KHCO3是环境影响的主要贡献者(84 %)。成本效益分析表明,K-FM-BC在各种吸附剂中具有优异的成本效益(96.28 g Cd/$)。这些结果表明,K-FM-BC在修复Cd(II)污染废水中具有很大的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Science
工程技术-工程:化工
CiteScore
7.50
自引率
8.50%
发文量
1025
审稿时长
50 days
期刊介绍:
Chemical engineering enables the transformation of natural resources and energy into useful products for society. It draws on and applies natural sciences, mathematics and economics, and has developed fundamental engineering science that underpins the discipline.
Chemical Engineering Science (CES) has been publishing papers on the fundamentals of chemical engineering since 1951. CES is the platform where the most significant advances in the discipline have ever since been published. Chemical Engineering Science has accompanied and sustained chemical engineering through its development into the vibrant and broad scientific discipline it is today.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


