Genetic modifiers and ascertainment drive variable expressivity of complex disorders
IF 42.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Variable expressivity of disease-associated variants implies a role for secondary variants that modify clinical features. We assessed the effects of modifier variants on the clinical outcomes of 2,455 individuals with primary variants. Among 124 families with the 16p12.1 deletion, distinct rare and common variant classes conferred risks for specific developmental features, including short tandem repeats for neurological defects. Network analysis suggested distinct mechanisms involving 16p12.1 genes and secondary variants specific to each proband. Within disease and population cohorts of 976 individuals with the 16p12.1 deletion, we found opposing effects of secondary variants on clinical features across ascertainments. Additional analysis of 1,479 probands with other primary variants, such as the 16p11.2 deletion and CHD8 variants, and 1,528 probands without primary variants showed that phenotypic associations differed by primary variant context and were influenced by synergistic interactions between primary and secondary variants. Our study provides a paradigm to dissect the personalized genomic architecture of complex disorders.
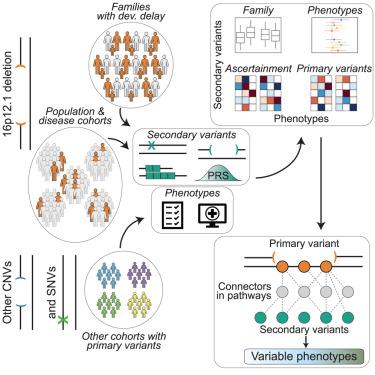
遗传修饰因子和确定驱动复杂疾病的可变表达
疾病相关变异的可变表达性意味着继发性变异可以改变临床特征。我们评估了修饰变异对2,455例原发性变异患者临床结果的影响。在124个16p12.1缺失的家族中,不同的罕见和常见变异类别赋予了特定发育特征的风险,包括神经缺陷的短串联重复序列。网络分析表明不同的机制涉及16p12.1基因和每个先证者特有的继发变异。在976名16p12.1缺失个体的疾病和人群队列中,我们发现继发变异对临床特征的相反影响。对1479个具有其他主要变异(如16p11.2缺失和CHD8变异)的先证和1528个没有主要变异的先证的进一步分析表明,表型关联因主要变异背景而异,并受到主要和次要变异之间的协同相互作用的影响。我们的研究为剖析复杂疾病的个性化基因组结构提供了一个范例。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell
生物-生化与分子生物学
CiteScore
110.00
自引率
0.80%
发文量
396
审稿时长
2 months
期刊介绍:
Cells is an international, peer-reviewed, open access journal that focuses on cell biology, molecular biology, and biophysics. It is affiliated with several societies, including the Spanish Society for Biochemistry and Molecular Biology (SEBBM), Nordic Autophagy Society (NAS), Spanish Society of Hematology and Hemotherapy (SEHH), and Society for Regenerative Medicine (Russian Federation) (RPO).
The journal publishes research findings of significant importance in various areas of experimental biology, such as cell biology, molecular biology, neuroscience, immunology, virology, microbiology, cancer, human genetics, systems biology, signaling, and disease mechanisms and therapeutics. The primary criterion for considering papers is whether the results contribute to significant conceptual advances or raise thought-provoking questions and hypotheses related to interesting and important biological inquiries.
In addition to primary research articles presented in four formats, Cells also features review and opinion articles in its "leading edge" section, discussing recent research advancements and topics of interest to its wide readership.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


