Disruption of P. falciparum amino acid transporter elevates intracellular proline and induces resistance to Prolyl-tRNA synthetase inhibitors
IF 7.2
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
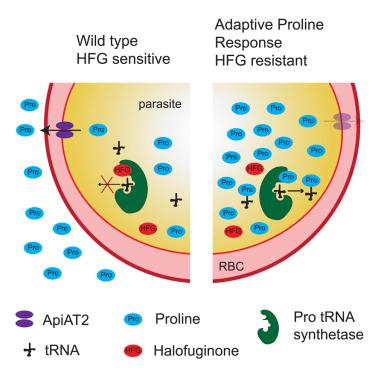
Plasmodium falciparum evades the antimalarial activity of proline-competitive prolyl-tRNA synthetase (PfProRS) inhibitors, such as halofuginone (HFG), by a resistance mechanism termed the adaptive proline response (APR). The APR is characterized by a marked elevation of intracellular proline following drug exposure. Contrary to initial expectations, the APR is not mediated by alterations in canonical proline metabolic pathways involving arginase (P. falciparum arginase [PfARG]) and ornithine aminotransferase (P. falciparum ornithine aminotransferase [PfOAT]). Instead, we identified loss-of-function mutations in the apicomplexan amino acid transporter 2 (P. falciparum apicomplexan amino acid transporter 2 [PfApiAT2]) as the primary genetic driver of this resistance phenotype. Importantly, reversion of these mutations to wild type effectively suppresses the APR, establishing PfApiAT2 as the molecular determinant of this resistance mechanism.The elucidation of the APR significantly advances our understanding of antimalarial drug resistance. By delineating the role of PfApiAT2 in this process, we establish critical insights for the development of strategies to circumvent PfProRS inhibitor resistance for future antimalarial therapies.
恶性疟原虫氨基酸转运体的破坏可提高细胞内脯氨酸并诱导对脯氨酸- trna合成酶抑制剂的抗性
恶性疟原虫通过一种被称为适应性脯氨酸反应(APR)的耐药机制,避开脯氨酸竞争性脯氨酸- trna合成酶(pfproors)抑制剂的抗疟活性,如卤富酮(HFG)。APR的特点是药物暴露后细胞内脯氨酸显著升高。与最初的预期相反,APR不是通过包括精氨酸酶(P. falciparum arginase [PfARG])和鸟氨酸转氨酶(P. falciparum ornithine aminotransferase [PfOAT])的典型脯氨酸代谢途径的改变介导的。相反,我们发现顶复合体氨基酸转运体2 (pfa)的功能缺失突变是这种抗性表型的主要遗传驱动因素。重要的是,将这些突变逆转为野生型有效地抑制了APR,确立了PfApiAT2作为这种抗性机制的分子决定因素。APR的阐明极大地促进了我们对抗疟药耐药性的认识。通过描述PfApiAT2在这一过程中的作用,我们为开发规避PfProRS抑制剂耐药性的策略建立了关键的见解,以用于未来的抗疟疾治疗。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell Chemical Biology
Biochemistry, Genetics and Molecular Biology-Molecular Medicine
CiteScore
14.70
自引率
2.30%
发文量
143
期刊介绍:
Cell Chemical Biology, a Cell Press journal established in 1994 as Chemistry & Biology, focuses on publishing crucial advances in chemical biology research with broad appeal to our diverse community, spanning basic scientists to clinicians. Pioneering investigations at the chemistry-biology interface, the journal fosters collaboration between these disciplines. We encourage submissions providing significant conceptual advancements of broad interest across chemical, biological, clinical, and related fields. Particularly sought are articles utilizing chemical tools to perturb, visualize, and measure biological systems, offering unique insights into molecular mechanisms, disease biology, and therapeutics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


