Targeting PIEZO1-TMEM16F Coupling to Mitigate Sickle Cell Disease Complications
IF 9.9
1区 医学
Q1 HEMATOLOGY
引用次数: 0
Abstract
A deeper understanding of sickle cell disease (SCD) pathophysiology is critical for identifying novel therapeutic targets. A hallmark of SCD is abnormal phosphatidylserine (PS) exposure on sickle red blood cells (RBCs), which contributes to anemia, thrombosis, and vaso-occlusive crises (VOC). However, the mechanisms underlying this excessive PS exposure remain unclear. Here, we identify TMEM16F, a Ca2+-activated lipid scramblase, as a key mediator of PS exposure downstream of Ca2+ influx through the mechanosensitive channel PIEZO1 in sickle RBCs. Electrophysiology, imaging, and flow cytometry reveal that deoxygenation-induced sickling activates PIEZO1, triggering Ca2+ entry, TMEM16F activation, and PS exposure. This cascade promotes PS+ microparticle release, thrombin generation, and RBC adhesion to endothelial cells. Notably, partial PIEZO1 inhibition with benzbromarone, an anti-gout drug, suppresses these effects. Our findings define a previously unrecognized mechanotransduction pathway in sickle RBCs and propose a unique therapeutic strategy to mitigate hypercoagulability and vaso-occlusion associated with SCD.
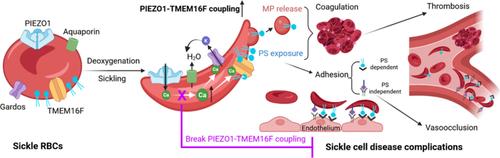
靶向PIEZO1-TMEM16F偶联减轻镰状细胞病并发症
深入了解镰状细胞病(SCD)的病理生理对确定新的治疗靶点至关重要。SCD的一个标志是镰状红细胞(rbc)中磷脂酰丝氨酸(PS)的异常暴露,这会导致贫血、血栓形成和血管闭塞危象(VOC)。然而,这种过度接触PS的机制尚不清楚。在这里,我们发现TMEM16F,一种Ca2+激活的脂质超高压酶,是镰状红细胞中通过机械敏感通道PIEZO1的Ca2+内流下游的PS暴露的关键介质。电生理、成像和流式细胞术显示,脱氧诱导的镰状细胞活化PIEZO1,触发Ca2+进入、TMEM16F激活和PS暴露。这个级联促进PS+微粒释放、凝血酶生成和红细胞粘附内皮细胞。值得注意的是,用抗痛风药物苯溴马龙局部抑制PIEZO1可以抑制这些作用。我们的发现在镰状红细胞中定义了一个以前未被认识的机械转导途径,并提出了一种独特的治疗策略来减轻与SCD相关的高凝性和血管闭塞性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
15.70
自引率
3.90%
发文量
363
审稿时长
3-6 weeks
期刊介绍:
The American Journal of Hematology offers extensive coverage of experimental and clinical aspects of blood diseases in humans and animal models. The journal publishes original contributions in both non-malignant and malignant hematological diseases, encompassing clinical and basic studies in areas such as hemostasis, thrombosis, immunology, blood banking, and stem cell biology. Clinical translational reports highlighting innovative therapeutic approaches for the diagnosis and treatment of hematological diseases are actively encouraged.The American Journal of Hematology features regular original laboratory and clinical research articles, brief research reports, critical reviews, images in hematology, as well as letters and correspondence.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


