A predator–prey interaction between myxobacteria and fungi mediated by outer membrane vesicles
IF 13
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
Introduction
Outer membrane vesicles (OMVs) of Gram-negative bacteria mediate diverse functions in natural ecosystems. As a keystone taxon in soil, myxobacteria produce OMVs for cargo packing and microbial predation. However, the roles of OMVs in the interactions of myxobacteria with fungi remain poorly understood.Objectives
This work aims to clarify the role of outer membrane vesicles in the interaction between myxobacteria and fungi and the regulatory mechanism during the interaction process.Methods
We found that OMV plays a significant role in the interaction between Myxococcus sp. MP20 and Verticillium dahilae (Vd). We further identified the antifungal metabolites in OMV and verified the regulatory mechanism of OMV in the model strain Myxococcus xanthus DK 1622.Results
We discover that Myxococcus sp. MP20 uses fungal networks and cotton root exudates for spatial dispersal. MP20 deploys the antifungal metabolites myxothiazole via OMVs to inhibit Vd growth by fusing the OMVs with fungal cells, thus restraining fungal invasion. Containment of myxothiazol within OMVs maintains an effective antifungal concentration on target cells. Furthermore, we demonstrate that the release of OMVs from MP20 was suppressed by iron via a newly discovered ABC transport system. In turn the lower number of OMVs reduced the antifungal behavior of MP20, suggesting that iron acquisition regulates OMVs-mediated competition between myxobacteria and fungi. Our findings thus unravel a novel antifungal tactic employed by myxobacteria to suppress fungi prey and control Verticillium wilt, which also provides new insights for understanding predator–prey interactions.
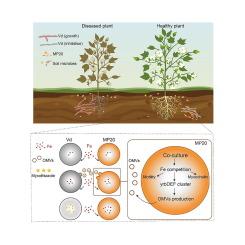
由外膜囊泡介导的黏菌和真菌之间的捕食者-猎物相互作用
革兰氏阴性菌外膜囊泡(OMVs)在自然生态系统中介导多种功能。黏菌是土壤中的一个重要分类群,其产生的黏菌可用于货物包装和微生物捕食。然而,omv在黏菌与真菌相互作用中的作用仍然知之甚少。目的阐明外膜囊泡在黏菌与真菌相互作用中的作用及其调控机制。方法发现OMV在黏液球菌(Myxococcus sp. MP20)与黄萎病菌(Verticillium dahilae, Vd)的相互作用中起重要作用。我们进一步鉴定了OMV的抗真菌代谢物,并验证了OMV对模式菌株黄粘球菌DK 1622的调控机制。结果黏液球菌MP20利用真菌网络和棉花根分泌物进行空间传播。MP20通过omv部署抗真菌代谢物粘噻唑,通过omv与真菌细胞融合抑制Vd生长,从而抑制真菌侵袭。在omv中控制粘噻唑可保持对靶细胞有效的抗真菌浓度。此外,我们证明了铁通过新发现的ABC转运系统抑制MP20中omv的释放。反过来,较少数量的omv降低了MP20的抗真菌行为,这表明铁获取调节了黏菌和真菌之间omv介导的竞争。因此,我们的发现揭示了黏菌抑制真菌猎物和控制黄萎病的一种新的抗真菌策略,这也为理解捕食者-猎物相互作用提供了新的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


