Machine learning real-time control of continuous granulation process
IF 5.2
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
The transition from traditional batch to continuous pharmaceutical manufacturing puts additional demands on the efficient process development and operation. The comprehensive understanding of complex interdependencies between critical process parameters (CPPs) and critical material attributes (CMAs) for the plants consisting of several unit operations is very challenging for process operators and experts. Therefore, the development of computational models is necessary to implement active process control and ensure a control state. Here, we present a machine-learning (ML) based approach to build a data-driven process model and to implement real-time process control for a continuous wet granulation line. The analysis of historical process data, where a set of experiments was performed for a targeted collection of new data, has allowed us to successfully build an ML kernel and to implement a control system for the granulation plant. Furthermore, to support the ML training process, the process data was extended with mechanistic models implemented as soft-sensors, resulting in a hybrid model architecture. The performed tests have shown that the proposed strategy and the developed ML system can be efficiently used to perform real-time control of the continuous plant and to achieve desired CMAs such as size and loss on drying of the final granules by adjusting CPPs.
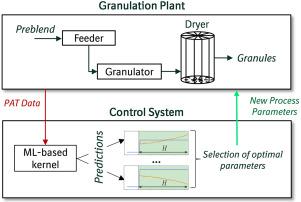
连续造粒过程的机器学习实时控制。
从传统的批量生产到连续生产的转变对高效的工艺开发和操作提出了额外的要求。对于由多个单元操作组成的工厂来说,全面理解关键工艺参数(CPPs)和关键材料属性(cma)之间复杂的相互依赖关系对工艺操作员和专家来说是非常具有挑战性的。因此,为了实现主动过程控制并保证控制状态,需要开发计算模型。在这里,我们提出了一种基于机器学习(ML)的方法来构建数据驱动的过程模型,并实现连续湿制粒线的实时过程控制。对历史过程数据的分析,其中一组实验是针对新数据的目标集合进行的,使我们能够成功地构建ML内核并实现造粒厂的控制系统。此外,为了支持训练过程,将过程数据扩展为实现为软传感器的机械模型,从而形成混合模型体系结构。实验表明,所提出的策略和所开发的ML系统可以有效地用于对连续装置进行实时控制,并通过调整CPPs来实现所需的cma,如最终颗粒的干燥尺寸和损失。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
10.70
自引率
8.60%
发文量
951
审稿时长
72 days
期刊介绍:
The International Journal of Pharmaceutics is the third most cited journal in the "Pharmacy & Pharmacology" category out of 366 journals, being the true home for pharmaceutical scientists concerned with the physical, chemical and biological properties of devices and delivery systems for drugs, vaccines and biologicals, including their design, manufacture and evaluation. This includes evaluation of the properties of drugs, excipients such as surfactants and polymers and novel materials. The journal has special sections on pharmaceutical nanotechnology and personalized medicines, and publishes research papers, reviews, commentaries and letters to the editor as well as special issues.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


