Additive-Switchable Alkylation/Alkenylation of 2-(Hetero)aryl-1-methylperimidines under Ruthenium Catalysis
IF 0.8
4区 化学
Q4 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
A switchable ruthenium-catalyzed C–H alkylation/alkenylation of 2-(hetero)aryl-substituted 1-methylperimidines with acrylic esters was developed, utilizing the perimidine moiety as an N-donor directing group. Employing commercially available, air-stable [RuCl2(p-cymene)]2 as the precatalyst affords the C–H functionalization products in high yields. The reaction selectivity is governed by the additive: Cu(OAc)2 promotes alkenylation, whereas AgTFA favors alkylation.
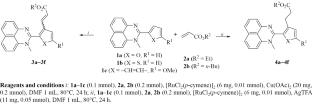
钌催化下2-(杂)芳基-1-甲基嘧啶的可加成切换烷基化/烯化反应
利用2-(杂)芳基取代的1-甲基吡啶部分作为n给体导向基团,制备了一种钌催化的2-(杂)芳基取代1-甲基吡啶与丙烯酸酯的C-H烷基化/烯化反应。采用市售的空气稳定的[RuCl2(p-聚伞烃)]2作为预催化剂,可获得高收率的碳氢功能化产物。反应选择性受添加剂控制:Cu(OAc)2促进烷基化,而AgTFA有利于烷基化。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
1.40
自引率
22.20%
发文量
252
审稿时长
2-4 weeks
期刊介绍:
Russian Journal of General Chemistry is a journal that covers many problems that are of general interest to the whole community of chemists. The journal is the successor to Russia’s first chemical journal, Zhurnal Russkogo Khimicheskogo Obshchestva (Journal of the Russian Chemical Society ) founded in 1869 to cover all aspects of chemistry. Now the journal is focused on the interdisciplinary areas of chemistry (organometallics, organometalloids, organoinorganic complexes, mechanochemistry, nanochemistry, etc.), new achievements and long-term results in the field. The journal publishes reviews, current scientific papers, letters to the editor, and discussion papers.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


