Development of MOF-808 based dispersive solid-phase extraction and ultra-high performance liquid chromatography tandem mass spectrometry for monitoring organophosphate diesters in urine
IF 6.1
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
Urinary organophosphate diesters (di-OPEs) serve as characteristic biomarkers for human exposure to organophosphate triesters (tri-OPEs). Existing sample pretreatment technologies for simultaneously extracting various di-OPEs in urine may be affected by the limited specific interactions and complicated urinary matrix interference, resulting in suboptimal recoveries. To establish a high-throughput and multi-target tool for monitoring urinary di-OPEs, zirconium-based metal organic framework MOF-808 was hydrothermally fabricated as dispersive solid-phase extraction (DSPE) adsorbents, and coupled with ultra-high performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) for quantification. MOF-808 exhibited maximum adsorption capacities of 108∼133 mg g−1 for target di-OPEs with a rapid adsorption equilibrium time of 30 min. The calculated adsorption energies between MOF-808 and di-OPEs ranged from −56.7 to −87.1 kJ mol−1, which were primarily driven by the synergistic effects of hydrogen bonding and π-π stacking. Under determined DSPE parameters, the developed DSPE-UPLC-MS/MS technology showed ideal linearity ranged from 0.05 to 50.0 ng mL−1, with limits of detection (LODs) of 0.02–0.45 ng mL−1, spiked recoveries of 75.6 %∼121.8 %, and matrix effects of 67.9 %∼121.0 %. This technology was successfully validated through the detection of twelve di-OPEs in 24-h pooled urine samples from college students, demonstrating the detection frequencies of 13.9 %–100 %, and specific gravity (SG)-adjusted median concentrations ranging from 0.09 to 7.62 ng mL−1. Notably, six urinary di-OPEs exhibited significantly higher levels in females than males (P < 0.05). Overall, this newly proposed DSPE-UPLC-MS/MS technology enabled simultaneous quantification of twelve di-OPEs in urine.
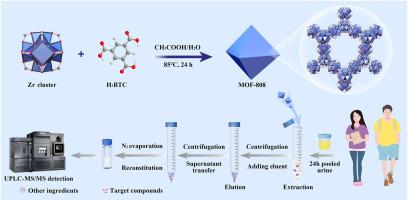
基于MOF-808分散固相萃取和超高效液相色谱串联质谱法监测尿液中有机磷二酯的研究
尿中有机磷二酯(di-OPEs)可作为人体暴露于有机磷三酯(tri-OPEs)的特征性生物标志物。现有的样品预处理技术可同时提取尿液中多种双opes,但由于特异性相互作用有限,且尿基质干扰复杂,导致回收率不理想。为了建立一种高通量、多靶点监测尿液中二氧opes的工具,采用水热法制备锆基金属有机骨架MOF-808作为分散固相萃取(DSPE)吸附剂,并结合超高效液相色谱-串联质谱(UPLC-MS/MS)进行定量分析。MOF-808对目标二opes的最大吸附量为108 ~ 133 mg g−1,快速吸附平衡时间为30 min。MOF-808与二opes的吸附能在−56.7 ~−87.1 kJ mol−1之间,主要由氢键和π-π堆积的协同作用驱动。在确定的DSPE参数下,建立的DSPE- uplc -MS/MS技术具有0.05 ~ 50.0 ng mL−1的理想线性范围,检出限(lod)为0.02 ~ 0.45 ng mL−1,加标回收率为75.6% ~ 121.8%,基质效应为67.9% ~ 121.0%。该技术通过对大学生24小时尿液样本中的12个diopes的检测成功验证,显示检测频率为13.9% - 100%,比重(SG)调整的中位浓度范围为0.09至7.62 ng mL - 1。值得注意的是,女性尿中6种dio - opes的水平明显高于男性(P < 0.05)。总的来说,这项新提出的DSPE-UPLC-MS/MS技术能够同时定量尿液中的12种二opes。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Talanta
化学-分析化学
CiteScore
12.30
自引率
4.90%
发文量
861
审稿时长
29 days
期刊介绍:
Talanta provides a forum for the publication of original research papers, short communications, and critical reviews in all branches of pure and applied analytical chemistry. Papers are evaluated based on established guidelines, including the fundamental nature of the study, scientific novelty, substantial improvement or advantage over existing technology or methods, and demonstrated analytical applicability. Original research papers on fundamental studies, and on novel sensor and instrumentation developments, are encouraged. Novel or improved applications in areas such as clinical and biological chemistry, environmental analysis, geochemistry, materials science and engineering, and analytical platforms for omics development are welcome.
Analytical performance of methods should be determined, including interference and matrix effects, and methods should be validated by comparison with a standard method, or analysis of a certified reference material. Simple spiking recoveries may not be sufficient. The developed method should especially comprise information on selectivity, sensitivity, detection limits, accuracy, and reliability. However, applying official validation or robustness studies to a routine method or technique does not necessarily constitute novelty. Proper statistical treatment of the data should be provided. Relevant literature should be cited, including related publications by the authors, and authors should discuss how their proposed methodology compares with previously reported methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


