In Situ Polymerized Gel Electrolytes on Na-Metal/Electrodes for High-Performance Sodium-Ion Batteries
IF 12.1
2区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
A solid sodium-ion conducting gel polymer electrolyte (GPE) is presented, directly synthesized on the sodium-metal/electrodes, as an alternative solid-like electrolyte to conventional liquid and ceramic electrolytes for high-performance sodium-ion batteries (SIBs). The GPE is obtained via an efficient synthesis protocol, wherein a mixture of liquid electrolyte (LE) and polymer precursors (PP) is in situ polymerized inside a prefabricated porous electrospun polyacrylonitrile (PAN) membrane, stabilized on either Na-metal or Na3V2(PO4) (NVP) electrode. The membrane's high porosity allows substantially high LE (≈60 vol%) confinement within its pores, which, following the polymerization, leads to a densely packed, mechanically and thermally stable amorphous gel. The GPE exhibited high sodium-ion conductivity (≈1 mS cm−1 ≈ LE ion conductivity) and transference number (0.63), wide electrochemical stability window (5.2 V), and excellent interfacial stability. The chemically stable GPE mitigated dendritic growth, leading to successive sodium-stripping/plating over 100s of cycles with low overpotentials at varying current densities. While the solid–electrolyte interphase (SEI) growth on the Na-electrode in the case of LE, studied using electrochemical impedance spectroscopy, reveals an unstable self-similar variation of growth with time (≈1 month), the GPE exhibits a stable SEI growth over longer durations of time (≈2 months). In (Na||Na3V2(PO4)3) configuration, i.e. sodium-metal batteries (SMBs) and symmetric Na3V2(PO4)3||Na3V2(PO4)3 batteries, the GPE shows remarkable cyclability, delivering consistent performance at 1C over 500–1000 cycles at room temperature. The diversity of GPE is also demonstrated via its remarkably stable cyclability over 100s of cycles with novel Sn-based alloy anodes, an upcoming alternative anode to hard carbons.
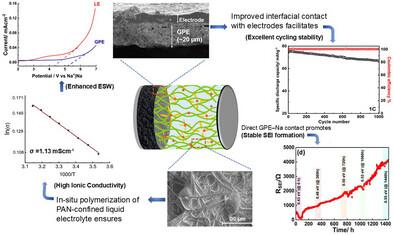
高性能钠离子电池用Na-Metal/电极原位聚合凝胶电解质
提出了一种直接在金属钠/电极上合成的固体钠离子导电凝胶聚合物电解质(GPE),作为高性能钠离子电池(sib)中传统液体电解质和陶瓷电解质的替代固体电解质。GPE是通过一种高效的合成方案获得的,其中液体电解质(LE)和聚合物前体(PP)的混合物在预制多孔电纺丝聚丙烯腈(PAN)膜内原位聚合,在na -金属或Na3V2(PO4) (NVP)电极上稳定。膜的高孔隙率允许在其孔内具有相当高的LE(≈60 vol%)限制,这在聚合之后,导致致密堆积,机械和热稳定的无定形凝胶。GPE具有高钠离子电导率(≈1 mS cm−1≈LE离子电导率)和转移数(0.63)、宽电化学稳定窗口(5.2 V)和优异的界面稳定性。化学稳定的GPE减缓了枝晶的生长,在不同的电流密度下以低过电位连续进行100次循环的钠剥离/镀。使用电化学阻抗谱研究LE情况下na电极上的固体电解质间相(SEI)生长显示出不稳定的自相似生长随时间变化(≈1个月),而GPE在较长时间内(≈2个月)表现出稳定的SEI生长。在(Na||Na3V2(PO4)3)配置中,即钠金属电池(smb)和对称Na3V2(PO4)3||Na3V2(PO4)3电池,GPE表现出显著的可循环性,在室温下,在1C下500-1000次循环中提供一致的性能。GPE的多样性还通过其在新型锡基合金阳极(即将成为硬碳的替代阳极)上超过100次循环的显着稳定的可循环性得到证明。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Small
工程技术-材料科学:综合
CiteScore
17.70
自引率
3.80%
发文量
1830
审稿时长
2.1 months
期刊介绍:
Small serves as an exceptional platform for both experimental and theoretical studies in fundamental and applied interdisciplinary research at the nano- and microscale. The journal offers a compelling mix of peer-reviewed Research Articles, Reviews, Perspectives, and Comments.
With a remarkable 2022 Journal Impact Factor of 13.3 (Journal Citation Reports from Clarivate Analytics, 2023), Small remains among the top multidisciplinary journals, covering a wide range of topics at the interface of materials science, chemistry, physics, engineering, medicine, and biology.
Small's readership includes biochemists, biologists, biomedical scientists, chemists, engineers, information technologists, materials scientists, physicists, and theoreticians alike.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


