Tandem confinement of sub-nanometer Pt–Cu sites in USY zeolite enables high NH3-SCO selectivity across an ultra-wide temperature window
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Selective catalytic oxidation of ammonia (NH3-SCO) to N2 is crucial for abating residual NH3 emissions, but existing catalysts struggle to combine low-temperature activity with high N2 selectivity due to competitive Pt-NO interactions. Here we design a tandem confinement catalyst by depositing sub-nanometer Pt clusters (∼0.72 nm) inside the supercages of a Cu-exchanged USY zeolite via atomic layer deposition (ALD), creating intimately paired Pt–Cu active sites. This confined Pt/Cu-USY ALD catalyst achieves >90 % NH3 conversion and >90 % N2 selectivity across an exceptionally broad 170-300°C window under simulated exhaust conditions, surpassing all previously reported Pt-based NH3-SCO catalysts. The simultaneously enhanced low-temperature activity and high-temperature N2 selectivity of the Pt/Cu-USY ALD catalyst can be attributed to the spatial confinement of Pt clusters within the Cu-USY framework, which leads to an expanded NO desorption window (150–350 °C). Detailed in situ studies further reveal that N2O4 species preferentially serve as NO storage intermediates on the Pt/Cu-USY ALD catalyst surface at low temperatures, exhibiting significantly lower formation barriers compared to free nitrates, which dominate as intermediates in conventional Pt–Cu systems. This effectively mitigates free nitrate–induced site poisoning and overcomes the typical activity–selectivity trade-offs observed in conventional Pt–Cu dual-site catalysts.
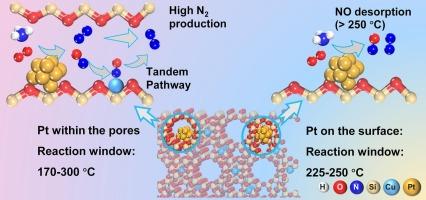
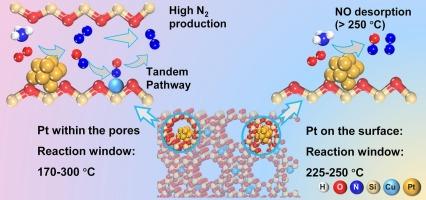
在USY沸石中,亚纳米级Pt-Cu位点的串联约束使NH3-SCO在超宽温度窗内具有高选择性
氨(NH3- sco)选择性催化氧化为N2对于减少残余NH3排放至关重要,但由于Pt-NO相互作用的竞争性,现有催化剂难以将低温活性与高N2选择性结合起来。在这里,我们设计了一种串联约束催化剂,通过原子层沉积(ALD)在cu交换的USY沸石的超笼内沉积亚纳米Pt簇(~ 0.72 nm),产生密切配对的Pt - cu活性位点。这种受限Pt/Cu-USY ALD催化剂在模拟排气条件下,在170-300°C的非常宽的窗口内实现了90% %的NH3转化率和90% %的N2选择性,超过了之前报道的所有基于Pt的NH3- sco催化剂。Pt/Cu-USY ALD催化剂的低温活性和高温N2选择性同时增强,可归因于Cu-USY框架内Pt簇的空间限制,从而扩大了NO解吸窗口(150-350 °C)。详细的原位研究进一步表明,在低温下,N2O4优先作为Pt/Cu-USY ALD催化剂表面的NO存储中间体,与传统Pt - cu体系中主要作为中间体的游离硝酸盐相比,表现出明显更低的形成障碍。这有效地减轻了游离硝酸盐引起的位点中毒,克服了传统Pt-Cu双位点催化剂中观察到的典型活性-选择性权衡。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


