Customized Solvation Structure for Stable and Safe Sodium‐Metal Batteries
IF 26
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Owing to the low cost and high abundance of sodium (Na), significant advancements are made in the field of Na‐based batteries, which are possible through the incorporation of lithium‐ion‐inspired electrodes with different electrolyte chemistries. However, the conventional carbonate electrolytes employed in such systems are flammable, thermally unstable, and prone to severe interfacial side reactions that compromise safety and shorten the overall battery cycle life. This study introduces a flame‐retardant cosolvent strategy, wherein trimethylsilyl phosphite (TMSPi) and nonafluorohexyltrimethoxysilane (NFTOS) are blended into a propylene carbonate (PC)‐based commercial electrolyte, yielding an novel electrolyte system (PCTN) with a tailor‐designed solvation structure. This PCTN electrolyte is found to self‐extinguish upon ignition, in addition to generating a robust NaSiO
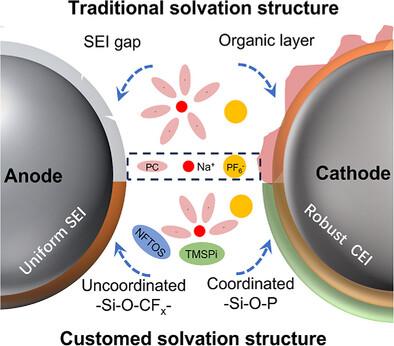
为稳定和安全的钠金属电池定制的溶剂化结构
由于钠(Na)的低成本和高丰度,在钠基电池领域取得了重大进展,这可以通过结合具有不同电解质化学性质的锂离子激发电极来实现。然而,在这种系统中使用的传统碳酸盐电解质是易燃的,热不稳定的,并且容易发生严重的界面副反应,从而危及安全性并缩短电池的整体循环寿命。本研究介绍了一种阻燃共溶剂策略,将三甲基硅基亚磷酸酯(TMSPi)和非氟己基三甲氧基硅烷(NFTOS)混合到碳酸丙烯酯(PC)基商业电解质中,得到一种具有定制溶剂化结构的新型电解质体系(PCTN)。该PCTN电解质被发现在点火时自熄,除了产生坚固的NaSiOx/NaF固体电解质间相和NaPOxFy主导的阴极电解质间相。因此,含有PCTN的Na|NaNi0.4Fe0.2Mn0.4O2电池在1℃下循环450次后仍保持90.3%的初始容量,优于商业电解质。即使在70°C的高工作温度下,使用PCTN进行100次循环后,容量保持率也达到93.2% (c.f,商用电解质的容量保持率为79.9%)。总的来说,这项工作展示了一种简单的、生产兼容的电解质设计,同时增强了电极-电解质界面的稳定性,为下一代高性能钠金属电池提供了一个潜在的系统。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Advanced Energy Materials
CHEMISTRY, PHYSICAL-ENERGY & FUELS
CiteScore
41.90
自引率
4.00%
发文量
889
审稿时长
1.4 months
期刊介绍:
Established in 2011, Advanced Energy Materials is an international, interdisciplinary, English-language journal that focuses on materials used in energy harvesting, conversion, and storage. It is regarded as a top-quality journal alongside Advanced Materials, Advanced Functional Materials, and Small.
With a 2022 Impact Factor of 27.8, Advanced Energy Materials is considered a prime source for the best energy-related research. The journal covers a wide range of topics in energy-related research, including organic and inorganic photovoltaics, batteries and supercapacitors, fuel cells, hydrogen generation and storage, thermoelectrics, water splitting and photocatalysis, solar fuels and thermosolar power, magnetocalorics, and piezoelectronics.
The readership of Advanced Energy Materials includes materials scientists, chemists, physicists, and engineers in both academia and industry. The journal is indexed in various databases and collections, such as Advanced Technologies & Aerospace Database, FIZ Karlsruhe, INSPEC (IET), Science Citation Index Expanded, Technology Collection, and Web of Science, among others.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


