Sm-doped RuO2 electrocatalysts for an acidic oxygen evolution reaction: enhanced activity and stability via electronic structure modulation and oxygen vacancy introduction
IF 3.3
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
Proton exchange membrane water electrolysis (PEMWE) is a promising hydrogen production technology, due to its high current density, high efficiency, and compact configuration. Enhancing the performance and stability of oxygen evolution reaction (OER) catalysts in acidic environments is crucial for advancing PEMWE. In this study, we developed a Sm-doped RuO2 (Sm-RuO2) electrocatalyst using the sol–gel method. This catalyst's large specific surface area and numerous active sites significantly enhance its activity and stability. Detailed studies show that Sm doping optimizes the electronic structure of RuO2, fine-tunes the adsorption free energy of reaction intermediates, and creates oxygen vacancies, boosting intrinsic activity and preventing Ru over-oxidation. The experimental results show that Sm-RuO2 follows the AEM and exhibits remarkable stability during the OER process. The stronger *OH adsorption onto the active sites accelerates the reaction. The representative Sm-RuO2 achieves an overpotential of 219 mV at 10 mA cm−2, outperforming undoped RuO2 (280 mV), and operates stably for 50 h. This work provides new insights into designing high-performance electrocatalysts through electronic structure and oxygen vacancy modulation.
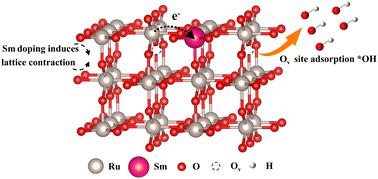
sm掺杂RuO2电催化剂的酸性析氧反应:通过电子结构调制和引入氧空位提高活性和稳定性
质子交换膜电解具有电流密度大、效率高、结构紧凑等优点,是一种很有前途的制氢技术。提高析氧反应(OER)催化剂在酸性环境中的性能和稳定性是推进PEMWE的关键。在这项研究中,我们采用溶胶-凝胶法开发了一种sm掺杂的RuO2 (Sm-RuO2)电催化剂。该催化剂具有较大的比表面积和众多的活性位点,大大提高了其活性和稳定性。详细研究表明,Sm掺杂优化了RuO2的电子结构,微调了反应中间体的吸附自由能,产生了氧空位,提高了Ru的固有活性,防止了Ru的过度氧化。实验结果表明,Sm-RuO2遵循AEM规律,在OER过程中表现出显著的稳定性。活性位点对*OH的吸附越强,反应速度越快。具有代表性的Sm-RuO2在10 mA cm−2下的过电位达到219 mV,优于未掺杂的RuO2 (280 mV),并稳定运行50 h。该研究为通过电子结构和氧空位调制设计高性能电催化剂提供了新的思路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Dalton Transactions
化学-无机化学与核化学
CiteScore
6.60
自引率
7.50%
发文量
1832
审稿时长
1.5 months
期刊介绍:
Dalton Transactions is a journal for all areas of inorganic chemistry, which encompasses the organometallic, bioinorganic and materials chemistry of the elements, with applications including synthesis, catalysis, energy conversion/storage, electrical devices and medicine. Dalton Transactions welcomes high-quality, original submissions in all of these areas and more, where the advancement of knowledge in inorganic chemistry is significant.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


