Unraveling the active site for CO2 hydrogenation to methanol over Cu/ZnO catalysts: insights from DFT and microkinetic modeling
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Extensive efforts have been devoted to the Cu/ZnO/Al2O3 catalyst system, which is widely applied in the industrial methanol synthesis from CO2. However, the nature of active sites remains controversial due to challenges in experimentally isolating contributions from different surface structures and the absence of standardized theoretical comparisons. Here, we systematically study several representative active sites on Cu/ZnO catalysts using DFT calculations and microkinetic modeling. The Cu/ZnO interface exhibits the highest intrinsic activity, with a turnover frequency (TOF) of 1.4 × 10–2 s−1 under typical reaction conditions, which is over four times higher than that of the Cu–Zn alloys and consistent with experimental observations. In contrast, the ZnO/Cu(111) overlayer is inactive due to prohibitively high barriers along all pathways. Reaction pathway analysis indicates that methanol synthesis proceeds primarily via the HCOO pathway, rather than through direct CO2 dissociation followed by CO hydrogenation. In addition, we find that a high H2O pressure in the system may further increase the surface coverage of OH* or O*, thereby hindering methanol formation by blocking active sites. These findings highlight the importance of considering adsorbate–adsorbate interactions in microkinetic modeling. Furthermore, tuning the coverage of specific intermediate species or the concentration of product can effectively enhance the reaction activity. This work offers theoretical insights into the fundamental origins of catalytic activity in Cu/ZnO systems and may facilitate the rational design of next-generation catalysts for methanol synthesis.

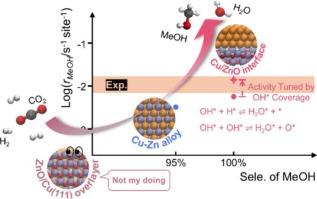
Cu/ZnO催化剂上CO2加氢制甲醇活性位点的揭示:来自DFT和微动力学模型的见解
Cu/ZnO/Al2O3催化剂体系在CO2工业甲醇合成中得到了广泛的应用。然而,由于在实验中分离不同表面结构的贡献和缺乏标准化理论比较的挑战,活性位点的性质仍然存在争议。本文采用DFT计算和微动力学模型,系统地研究了Cu/ZnO催化剂上几个具有代表性的活性位点。Cu/ZnO界面表现出最高的固有活性,在典型反应条件下,转换频率(TOF)为1.4 × 10-2 s−1,是Cu - zn合金的4倍以上,与实验观察结果一致。相比之下,ZnO/Cu(1 1 1)覆盖层由于沿所有途径的高阻而无活性。反应途径分析表明,甲醇的合成主要是通过HCOO途径进行的,而不是通过直接的CO2解离和CO加氢。此外,我们发现系统中较高的H2O压力可能会进一步增加OH*或O*的表面覆盖,从而通过阻断活性位点阻碍甲醇的形成。这些发现强调了在微动力学建模中考虑吸附物-吸附物相互作用的重要性。此外,调整特定中间物质的覆盖或产物的浓度可以有效地提高反应活性。这项工作为Cu/ZnO体系催化活性的基本起源提供了理论见解,并可能促进下一代甲醇合成催化剂的合理设计。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


