Synergistic acid–base interplays: Zeolite-catalyzed CH3Cl conversion to light olefins boosted by MgO-driven CH3Cl spillover
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
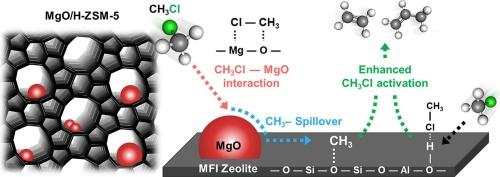
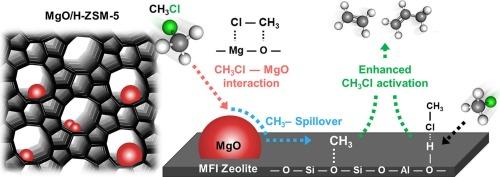
Chloromethane (CH3Cl), a reactive C1 molecule, has been underexplored compared to traditional C1 molecules like CO, CO2, CH4, and CH3OH that have long been at the center of C1 chemistry. Herein, the catalytic chloromethane–to–olefin (CMTO) reaction was investigated with zeolite-based heterogeneous acid catalysts. H-ZSM-5 zeolites with controlled Si/Al ratios were investigated, revealing that CH3Cl conversion, catalyst lifetime, and light olefin () selectivity depended on zeolite acidity and MgO addition. While MgO alone enabled CH3Cl activation via Lewis acid–base interactions, its independent catalytic play pales compared to the superior performance of zeolites. However, balancing the zeolite acidity with MgO loading doubled or even tripled CH3Cl conversion compared to the independent performance of zeolites, achieving nearly 100 % CH3Cl conversion with 86.5 % selectivity without deactivation. In-situ diffuse reflectance infrared Fourier transform spectroscopy showed that both zeolite acid sites and MgO activated CH3Cl simultaneously, during which MgO induced a CH3Cl spillover to the zeolite acidic site, resulting in the boosted CH3Cl conversion. This study establishes a new road to efficient and selective C1 molecular conversion using CH3Cl boosted by CH3Cl spillover, which would be extended to methyl spillover-mediated catalytic conversion to various chemicals.
协同酸碱相互作用:沸石催化的CH3Cl转化为轻烯烃由mgo驱动的CH3Cl溢出
氯甲烷(CH3Cl)是一种反应性的C1分子,与传统的C1分子(如CO、CO2、CH4和CH3OH)相比,一直处于C1化学的中心,对它的研究还不够充分。以沸石为催化剂,研究了氯甲烷制烯烃(CMTO)的催化反应。研究了控制Si/Al比的H-ZSM-5分子筛,发现分子筛酸度和MgO添加量对CH3Cl转化率、催化剂寿命和轻烯烃(C2-4=)选择性都有影响。虽然MgO单独通过路易斯酸碱相互作用激活了CH3Cl,但它的独立催化作用与沸石的优越性能相比相形见绌。然而,与沸石的独立性能相比,平衡MgO负载的沸石酸度使CH3Cl转化率增加了一倍甚至三倍,在不失活的情况下实现了接近100% %的CH3Cl转化率和86.5 %的C2-4=选择性。原位漫反射红外傅里叶变换光谱分析表明,沸石酸性位点和MgO同时激活CH3Cl, MgO诱导CH3Cl溢出到沸石酸性位点,导致CH3Cl转化率提高。本研究开辟了一条利用CH3Cl外溢促进高效、选择性转化C1分子的新途径,并将其扩展到甲基外溢介导的多种化学品催化转化。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


