Regulation of remote sites to enhance Pt activity in the hydrogenation of sulfur-containing nitroarenes
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Outer-sphere catalysis, commonly associated with enzyme and homogeneous catalysis, utilizes remote sites to drive reactions and is particularly effective for reactants that are toxic to the catalyst. However, this approach has been rarely explored in heterogeneous catalysis. In this study, we demonstrate the use of remote sites to enhance the activity of Pt nanoparticles (NPs) in the hydrogenation of sulfur-containing nitroarenes. Catalysts with Pt and MoO3 co-deposited on TiO2 efficiently catalyze the hydrogenation of 5-nitrobenzothiazole (NBZ) under mild conditions, achieving a conversion rate of 5448 molNBZ · molPt−1 · h−1, the highest reported to date, even with ppm levels of Pt. The optimized Pt density (∼3 NPs per 1 × 104 nm2) on the catalyst was found to favor HxMoO3 mediated sequential H transfer from Pt to Mo and subsequently to the substrate. While higher Pt density may enhance H transfer to Mo, the hydrogenation process becomes limited by the availability of HxMoO3 for further H transfer to the substrate, revealing the intrinsic reason for the high activity of catalysts with ppm Pt. A direct transfer of intercalated H in HxMoO3 to the substrate, rather than solvent-mediated proton-coupled electron transfer (PCET) dominating the hydrogenation, was observed and confirmed through solvent isotope kinetic effects and solvent studies. Tuning remote sites on solid catalysts offers a promising strategy for developing catalysts with the minimize the use of precious metals in the hydrogenation of strongly coordinating reactants.
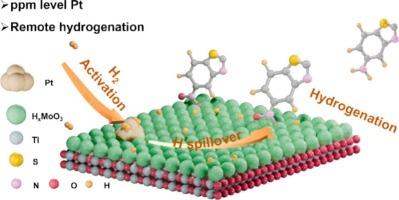
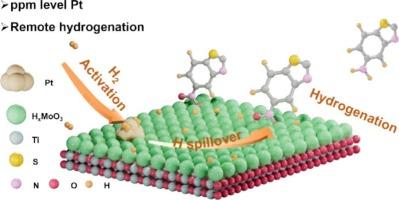
调控远程位点以增强含硫硝基芳烃加氢过程中的铂活性
外球催化通常与酶和均相催化有关,利用远程位点驱动反应,对对催化剂有毒的反应物特别有效。然而,这种方法在多相催化中很少被探索。在这项研究中,我们展示了使用远程位点来增强Pt纳米颗粒(NPs)在含硫硝基arenes加氢中的活性。Pt和MoO3共沉积在TiO2上的催化剂在温和的条件下有效地催化5-硝基苯并噻唑(NBZ)的加氢,即使在ppm的Pt水平下,转化率也达到5448 molNBZ·molPt−1·h−1,这是迄今为止报道的最高水平。催化剂上优化的Pt密度(每1 × 104 nm2)有利于HxMoO3介导的从Pt到Mo的顺序h转移。虽然较高的Pt密度可以促进氢向Mo的转移,但氢化过程受到HxMoO3进一步向底物转移氢的可用性的限制,这揭示了ppm Pt催化剂高活性的内在原因。通过溶剂同位素动力学效应和溶剂研究,观察并证实了HxMoO3中插入的氢直接向底物转移,而不是溶剂介导的质子耦合电子转移(PCET)主导氢化过程。调整固体催化剂上的远程位点为开发催化剂提供了一个有前途的策略,在强配位反应物的氢化过程中最大限度地减少贵金属的使用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


