CeO2/H-SSZ-13 composite NH3-SCR catalysts achieve unprecedented high-temperature N2 selectivity
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
A series of CeO2/H-SSZ-13 composite catalysts with CeO2 contents ranging from 5 to 50 wt% are investigated. The catalysts are characterized using various techniques, including XRD, SEM, TEM, XPS, H2-TPR, Raman spectroscopy and chemical titrations coupled with TPD and DRIFTS. Their catalytic performance is evaluated through steady-state NH3-SCR. DFT calculations are also applied to supplement experimental data. The results demonstrate that the synergistic interactions between the redox domain (CeO2) and the acid domain (H-SSZ-13) render the composite catalysts with excellent medium-to-high-temperature deNOx efficiency. Notably, the formation of the unwanted N2O side product is completely eliminated over these composite catalysts. By systematically regulating redox and acid functions, it is revealed that CeO2 serves as the key active component for the rate-determining NO activation step, while H-SSZ-13 facilitates NH3 adsorption and the decomposition of reactive intermediates. Based on detailed studies of SCR kinetics, N2O formation, chemical trapping, as well as DFT calculations, it is proposed that the gaseous nitrite precursor HONO acts as the key reaction intermediate.
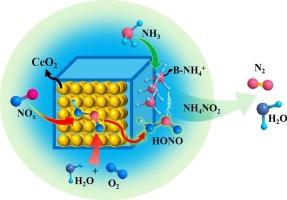
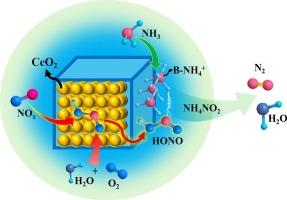
CeO2/H-SSZ-13复合NH3-SCR催化剂实现了前所未有的高温N2选择性
研究了一系列CeO2含量为5 ~ 50 wt%的CeO2/H-SSZ-13复合催化剂。采用XRD、SEM、TEM、XPS、H2-TPR、拉曼光谱、化学滴定、TPD和DRIFTS等技术对催化剂进行了表征。通过稳态NH3-SCR评价了它们的催化性能。DFT计算也用于补充实验数据。结果表明,氧化还原域(CeO2)和酸域(H-SSZ-13)之间的协同作用使复合催化剂具有优异的中高温脱氧效率。值得注意的是,在这些复合催化剂上完全消除了不必要的N2O副产物的形成。通过系统调节氧化还原和酸功能,揭示了CeO2是决定速率的NO活化步骤的关键活性组分,而H-SSZ-13促进NH3吸附和反应中间体分解。基于SCR动力学、N2O生成、化学捕集以及DFT计算的详细研究,提出气态亚硝酸盐前体HONO作为关键反应中间体。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


