Mycotoxin Alternariol exposure promotes endoplasmic reticulum stress-induced hepatotoxicity to exacerbate chronic liver injury
IF 7.3
2区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
Abstract
As a mycotoxin ubiquitously detected in global food supplies, Alternariol (AOH) has garnered significant attention due to its documented health risks. However, hepatotoxic effect of chronic exposure to AOH at environmental levels remains poorly characterized. Herein, we aim to investigate the long-term effects of dietary AOH exposure on murine liver and key signaling event through integrated in vivo and in vitro models. AOH exposure significantly induced dose-dependent hepatopathy characterized by liver injury, inflammatory cell infiltration, and liver fibrosis. RNA-sequencing analyses further indicated that the pivotal molecular mechanism linking the hepatotoxic effect of AOH were endoplasmic reticulum (ER) stress-mediated apoptosis via PERK/ATF4/CHOP signaling cascade. In vitro models confirmed AOH promoted the PERK/ATF4/CHOP signaling activation and subsequent apoptosis in primary murine hepatocytes and human hepatocyte cell line THLE-2. Pharmacological intervention studies demonstrated that both TUDCA (ER stress inhibitor) and GSK2606414 (PERK inhibitor) effectively mitigated AOH-induced cytotoxicity in hepatocytes. Notably, TUDCA administration demonstrated therapeutic efficacy in mitigating AOH-induced hepatic pathology in mice. Thus, we delineate ER stress as central mechanism driving AOH-induced hepatotoxicity and validate PERK cascade inhibition as viable protective strategy. These findings fundamentally refine risk assessment framework for mycotoxin exposure while proposing novel molecular targets for therapeutic intervention against environmental hepatotoxicants.
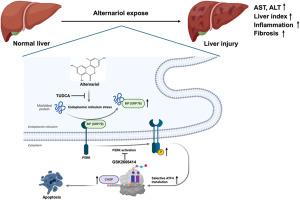
真菌毒素交替胺暴露促进内质网应激性肝毒性,加重慢性肝损伤
作为一种在全球食品供应中普遍检测到的真菌毒素,Alternariol (AOH)因其记录在案的健康风险而引起了极大的关注。然而,慢性暴露于环境水平的AOH的肝毒性作用仍然不清楚。本研究旨在通过体内和体外综合模型,研究饮食中AOH暴露对小鼠肝脏和关键信号事件的长期影响。AOH暴露显著诱导以肝损伤、炎症细胞浸润和肝纤维化为特征的剂量依赖性肝病。rna测序分析进一步表明,AOH肝毒性作用的关键分子机制是内质网应激介导的PERK/ATF4/CHOP信号级联凋亡。体外模型证实,AOH促进原代小鼠肝细胞和人肝细胞THLE-2的PERK/ATF4/CHOP信号激活和随后的凋亡。药理干预研究表明,TUDCA (ER应激抑制剂)和GSK2606414 (PERK抑制剂)均能有效减轻aoh诱导的肝细胞毒性。值得注意的是,TUDCA在减轻aoh诱导的小鼠肝脏病理方面显示出治疗效果。因此,我们将内质网应激描述为驱动aoh诱导的肝毒性的主要机制,并验证PERK级联抑制是可行的保护策略。这些发现从根本上完善了霉菌毒素暴露的风险评估框架,同时提出了针对环境肝毒物的治疗干预的新分子靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Environmental Pollution
环境科学-环境科学
CiteScore
16.00
自引率
6.70%
发文量
2082
审稿时长
2.9 months
期刊介绍:
Environmental Pollution is an international peer-reviewed journal that publishes high-quality research papers and review articles covering all aspects of environmental pollution and its impacts on ecosystems and human health.
Subject areas include, but are not limited to:
• Sources and occurrences of pollutants that are clearly defined and measured in environmental compartments, food and food-related items, and human bodies;
• Interlinks between contaminant exposure and biological, ecological, and human health effects, including those of climate change;
• Contaminants of emerging concerns (including but not limited to antibiotic resistant microorganisms or genes, microplastics/nanoplastics, electronic wastes, light, and noise) and/or their biological, ecological, or human health effects;
• Laboratory and field studies on the remediation/mitigation of environmental pollution via new techniques and with clear links to biological, ecological, or human health effects;
• Modeling of pollution processes, patterns, or trends that is of clear environmental and/or human health interest;
• New techniques that measure and examine environmental occurrences, transport, behavior, and effects of pollutants within the environment or the laboratory, provided that they can be clearly used to address problems within regional or global environmental compartments.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


