Ultra-high concentration low-viscosity subcutaneous antibody formulations using ionic liquids
IF 11.5
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
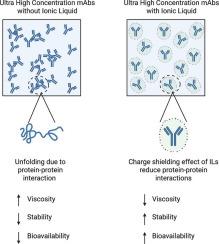
Monoclonal antibodies (mAbs) are the single largest class of therapeutics used in various indications including oncology, immunology, and neurology. Given their high prevalence, the development of subcutaneously administrable mAb formulations via conventional needles is an urgent and unmet need. Subcutaneous mAb formulations need to meet several stringent requirements, including high concentrations to enable the delivery of therapeutic doses through small volumes, low viscosity to allow self-administration, and high shelf stability, all of which are inherently linked to protein-protein interactions, which have collectively limited mAb concentrations in current commercial formulations to ~150 mg/ml. Here, we report the use of ionic liquids (ILs) to mitigate protein-protein interactions to formulate antibodies at an ultra-high concentration in excess of 200 mg/ml. In addition to solubilizing antibodies at such high concentrations, IgG-IL formulations maintained a viscosity below the injectable threshold of 20 cP, remained stable at even room temperature and 37 °C, and exhibited improved bioavailability compared to saline formulations upon subcutaneous administration.
使用离子液体的超高浓度低粘度皮下抗体配方
单克隆抗体(mab)是最大的一类用于各种适应症的治疗药物,包括肿瘤学,免疫学和神经学。鉴于其高流行率,通过传统针头开发皮下给药单抗制剂是一项迫切且未得到满足的需求。皮下单抗制剂需要满足几个严格的要求,包括高浓度,以便通过小体积递送治疗剂量,低粘度,允许自我给药,以及高货架稳定性,所有这些都与蛋白质-蛋白质相互作用有关,这些共同限制了当前商业制剂中的单抗浓度~150 mg/ml。在这里,我们报告使用离子液体(ILs)来减轻蛋白质之间的相互作用,以形成超过200 mg/ml的超高浓度的抗体。除了在如此高的浓度下溶解抗体外,IgG-IL制剂保持粘度低于20 cP的注射阈值,在室温和37 °C下保持稳定,并且与皮下给药的生理盐水制剂相比,表现出更好的生物利用度。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Controlled Release
医学-化学综合
CiteScore
18.50
自引率
5.60%
发文量
700
审稿时长
39 days
期刊介绍:
The Journal of Controlled Release (JCR) proudly serves as the Official Journal of the Controlled Release Society and the Japan Society of Drug Delivery System.
Dedicated to the broad field of delivery science and technology, JCR publishes high-quality research articles covering drug delivery systems and all facets of formulations. This includes the physicochemical and biological properties of drugs, design and characterization of dosage forms, release mechanisms, in vivo testing, and formulation research and development across pharmaceutical, diagnostic, agricultural, environmental, cosmetic, and food industries.
Priority is given to manuscripts that contribute to the fundamental understanding of principles or demonstrate the advantages of novel technologies in terms of safety and efficacy over current clinical standards. JCR strives to be a leading platform for advancements in delivery science and technology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


