Innate immune mechanisms underlying the efficacy of a next-generation nanoparticle-based vaccine against opioid use disorders
IF 11.5
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
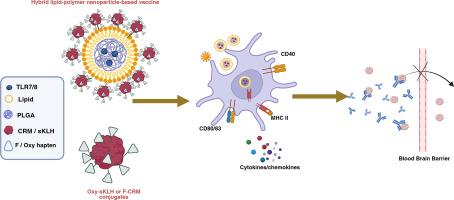
Since 1999, the opioid use disorder (OUD) and overdose epidemic has claimed millions of lives with ~3 million people currently diagnosed with OUD and ~ 100,000 fatal overdoses occurring annually. Curbing this public health crisis requires accelerating the translation of innovative, effective, and safe treatments. Our team is advancing vaccines against a variety of opioids as a preventative and therapeutic strategy against OUD and overdose. Previously, we reported that a hybrid lipid-polymer nanoparticle (HLPNP)-based vaccine targeting oxycodone demonstrated superior immunogenicity and efficacy in mice compared to an equivalent conjugate vaccine formulated in aluminum salts. Here, we extended our investigation of the HLPNP-based oxycodone vaccine to rats and applied the HLPNP platform to a lead vaccine targeting fentanyl and its analogs. Additionally, this study tested whether HLPNP offer a suitable delivery system for vaccines adjuvanted with the toll-like receptor 7 and 8 (TLR7/8) agonist R848. This study assessed vaccine efficacy in blocking opioid-induced antinociception, respiratory/cardiovascular depression, and drug distribution to the brain. Innate immune responses to the anti-oxycodone nanovaccines were tested in murine macrophage and dendritic cell (DC) lines using cellular activation co-stimulatory/maturation markers. Cytokine profiles and immune cell activation in response to anti-fentanyl nanovaccines were assessed in human whole blood using multiplex cytokine assays paired with flow cytometry. Results demonstrate that HLPNP-formulated oxycodone vaccines exhibit improved efficacy in rats and superior activation of macrophage and DC compared to aluminum formulated conjugate vaccines. While the HLPNP-based fentanyl vaccine showed diminished efficacy relative to the unformulated vaccine in rats, adding R848 to the HLPNP formulation rescued its efficacy. These comprehensive immune profiling advance our understanding of OUD vaccines, providing a rational blueprint for next-generation vaccine design.
基于纳米颗粒的新一代阿片类药物使用障碍疫苗有效性的先天免疫机制
自1999年以来,阿片类药物使用障碍和过量流行夺去了数百万人的生命,目前约有300万人被诊断患有阿片类药物使用障碍,每年发生 ~ 10万例致命过量。遏制这一公共卫生危机需要加快转化创新、有效和安全的治疗方法。我们的团队正在推进针对各种阿片类药物的疫苗,作为预防和治疗OUD和过量的策略。先前,我们报道了一种基于混合脂质-聚合物纳米颗粒(HLPNP)的靶向氧可酮疫苗,与铝盐配制的等效结合疫苗相比,在小鼠中表现出更好的免疫原性和有效性。在这里,我们将基于HLPNP的羟考酮疫苗的研究扩展到大鼠,并将HLPNP平台应用于针对芬太尼及其类似物的先导疫苗。此外,本研究还测试了HLPNP是否为toll样受体7和8 (TLR7/8)激动剂R848佐剂的疫苗提供了合适的递送系统。本研究评估了疫苗在阻断阿片类药物诱导的抗痛觉、呼吸/心血管抑制和药物向大脑分布方面的功效。利用细胞激活共刺激/成熟标记物,在小鼠巨噬细胞和树突状细胞(DC)系中测试了抗氧可酮纳米疫苗的先天免疫应答。细胞因子谱和免疫细胞活化对抗芬太尼纳米疫苗的反应在人全血中进行评估,使用多重细胞因子测定与流式细胞术配对。结果表明,与铝配制的结合疫苗相比,hlpnp配制的羟考酮疫苗在大鼠中表现出更高的效力,并具有更强的巨噬细胞和DC活化能力。在大鼠中,与未配制的疫苗相比,以HLPNP为基础的芬太尼疫苗的疗效有所降低,但在HLPNP配方中加入R848可以恢复其疗效。这些全面的免疫分析促进了我们对OUD疫苗的理解,为下一代疫苗设计提供了合理的蓝图。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Controlled Release
医学-化学综合
CiteScore
18.50
自引率
5.60%
发文量
700
审稿时长
39 days
期刊介绍:
The Journal of Controlled Release (JCR) proudly serves as the Official Journal of the Controlled Release Society and the Japan Society of Drug Delivery System.
Dedicated to the broad field of delivery science and technology, JCR publishes high-quality research articles covering drug delivery systems and all facets of formulations. This includes the physicochemical and biological properties of drugs, design and characterization of dosage forms, release mechanisms, in vivo testing, and formulation research and development across pharmaceutical, diagnostic, agricultural, environmental, cosmetic, and food industries.
Priority is given to manuscripts that contribute to the fundamental understanding of principles or demonstrate the advantages of novel technologies in terms of safety and efficacy over current clinical standards. JCR strives to be a leading platform for advancements in delivery science and technology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


