Synergistic effect of nitrogen-vacancy generation and S-doping within g-C3N4: S-scheme homojunction photocatalysts for effectual NH3 production upon simulated solar light
IF 5.4
3区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
The photocatalytic production of ammonia from air nitrogen and water is green and sustainable, because the primary resources are available and abundant. In the present research, binary nitrogen-vacancy-rich g-C3N4/S-doped g-C3N4 (abbreviated as NvrGCN/S-GCN) nanocomposites were synthesized by an easy method and employed for N2 photofixation reaction. The amount of ammonia produced by the optimum NvrGCN/S-GCN nanocomposite was 31,824 μmol/L.g, which was almost 62, 3.2, and 3.3 times more than GCN, NvrGCN, and S-GCN photocatalysts, respectively. The S-type homojunction formed between NvrGCN and S-GCN counterparts is responsible for the promoted surface area, prolonged life for charge carriers, and less resistance for charge migration, which altogether collaborated in the improvement of nitrogen photofixation reaction toward ammonia production. To gain more insights about the reaction mechanism, the effects of light, water, air, solution pH, electrons, and protons on the ammonia production rate were explored. Furthermore, the amount of nitrate, nitrite, and hydrazine produced in the reaction media was assayed. The results of this research provides a straightforward procedure for fabrication of homojunction photocatalysts for ammonia production from nitrogen gas and water.
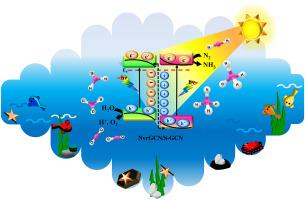
g-C3N4: s方案均结光催化剂中氮空位生成和s掺杂对模拟太阳光照下NH3有效生成的协同效应
空气氮和水的光催化制氨是绿色和可持续的,因为主要资源是可利用的和丰富的。本研究采用简单的方法合成了二元富氮空位g-C3N4/ s掺杂g-C3N4(简称NvrGCN/S-GCN)纳米复合材料,并将其用于N2光固定反应。最佳NvrGCN/S-GCN纳米复合材料产氨量为31824 μmol/L。g,分别是GCN、NvrGCN和S-GCN光催化剂的62倍、3.2倍和3.3倍。NvrGCN与S-GCN之间形成的s型同质结提高了载体的表面积,延长了载流子的寿命,降低了电荷迁移的阻力,共同促进了氮光合作用对制氨的作用。为了进一步了解反应机理,研究了光、水、空气、溶液pH、电子和质子对氨生成速率的影响。此外,还测定了在反应介质中产生的硝酸盐、亚硝酸盐和肼的量。本研究结果为制备氮气和水制氨的均结光催化剂提供了一种简单的方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganic Chemistry Communications
化学-无机化学与核化学
CiteScore
5.50
自引率
7.90%
发文量
1013
审稿时长
53 days
期刊介绍:
Launched in January 1998, Inorganic Chemistry Communications is an international journal dedicated to the rapid publication of short communications in the major areas of inorganic, organometallic and supramolecular chemistry. Topics include synthetic and reaction chemistry, kinetics and mechanisms of reactions, bioinorganic chemistry, photochemistry and the use of metal and organometallic compounds in stoichiometric and catalytic synthesis or organic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


