Preparation of a Sn/NiO sensor and its charge transfer mechanism in NH3 detection application
IF 5.4
3区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
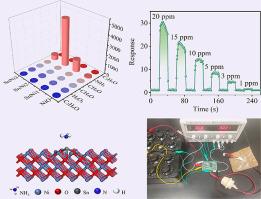
A high-performance NH3 sensor based on Sn/NiO was prepared by the hydrothermal method and applied to detect NH3 concentration in exhaled air. Experimental results showed that the Sn/NiO-based NH3 sensor has a low detection limit of 1 ppm and a response/recovery time of 2.8 s/2.0 s when detecting 10 ppm NH3. The enhanced gas sensitivity of the Sn/NiO NH3 sensor was mainly attributed to Sn doping. This doping increased the proportion of Ni3+ ions in NiO through the charge compensation effectIncreased Ni3+ ions can adsorb more oxygen vacancies and oxygen radicals. In addition, first-principles calculations explain the charge transfer mechanism of NH3 molecules during the adsorption process. In this process, NH3 molecules lose electrons and transfer them to the metal orbitals of Sn/NiO. Electron transfer increases the current of Sn/NiO during NH3 detection. Finally, a visual circuit was designed to determine whether the NH3 concentration in the breath test exceeded the human health standard. This provided a feasible solution for monitoring human health conditions by detecting the NH3 concentration in the breath.
Sn/NiO传感器的制备及其在NH3检测中的电荷转移机理
采用水热法制备了一种基于Sn/NiO的高性能NH3传感器,并将其应用于呼出空气中NH3浓度的检测。实验结果表明,该传感器在检测10 ppm NH3时的响应/恢复时间为2.8 s/2.0 s,检测限低至1 ppm。Sn/NiO NH3传感器的气敏性增强主要是由于Sn的掺杂。这种掺杂通过电荷补偿效应增加了Ni3+离子在NiO中的比例,增加的Ni3+离子可以吸附更多的氧空位和氧自由基。此外,第一性原理计算解释了NH3分子在吸附过程中的电荷转移机制。在这个过程中,NH3分子失去电子并将它们转移到Sn/NiO的金属轨道上。在NH3检测过程中,电子转移增加了Sn/NiO的电流。最后,设计了一个视觉回路来判断呼吸测试中的NH3浓度是否超过人体健康标准。这为通过检测呼吸中NH3浓度监测人体健康状况提供了一种可行的解决方案。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganic Chemistry Communications
化学-无机化学与核化学
CiteScore
5.50
自引率
7.90%
发文量
1013
审稿时长
53 days
期刊介绍:
Launched in January 1998, Inorganic Chemistry Communications is an international journal dedicated to the rapid publication of short communications in the major areas of inorganic, organometallic and supramolecular chemistry. Topics include synthetic and reaction chemistry, kinetics and mechanisms of reactions, bioinorganic chemistry, photochemistry and the use of metal and organometallic compounds in stoichiometric and catalytic synthesis or organic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


