Unveiling synergistic effect of potassium permanganate and potassium ferrate to manufacture magnetic biochar by low-temperature pyrolysis for efficient adsorption of tetracycline and copper
IF 4.3
2区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
Pyrolysis impregnation of magnetic iron is a promising strategy for preparation of magnetic biochar, but high temperature is usually required to produce magnetic iron species. Herein, a novel co-oxidative pyrolysis using potassium permanganate and potassium ferrate was explored for synthesis of magnetic biochar at 250–400 °C. Additionally, the magnetic biochar was applied to remove tetracycline and copper(II) from aqueous solution. This co-oxidative pyrolysis successfully impregnated magnetite, zero-valent iron, and manganese ferrite into carbon matrix with manganese oxides at 250 °C. Meanwhile, porous structures were created, accompanying by abundant oxygen groups. This magnetic biochar displayed 720.7 and 267.9 mg/g adsorption capacities toward tetracycline and copper(II) at 35 °C correspondingly, with 21.79 emu/g saturation magnetization. High adsorption capability was still achieved in real water matrices, showing great potential of the magnetic biochar for wastewater treatment in industry. The adsorption could be primarily contributed to hydrogen bond/complexation/π-π interaction/pore filling for tetracycline and cation exchange/complexation/electrostatic interaction/pore filling for copper(II). The success in fabrication of highly adsorptive magnetic biochar by potassium permanganate and potassium ferrate co-oxidative pyrolysis at low temperature, demonstrating oxidative magnetization was a promising technology for synthesis of high performance magnetic biochar with low energy consumption.
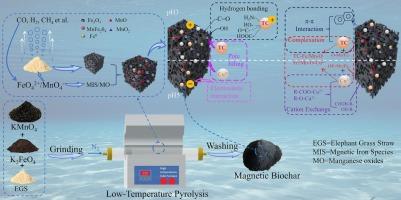
揭示高锰酸钾和高铁酸钾在低温热解制备磁性生物炭中的协同作用,以高效吸附四环素和铜
热解浸渍磁性铁是制备磁性生物炭的一种很有前途的方法,但制备磁性铁通常需要较高的温度。本文探索了高锰酸钾和高铁酸钾在250-400 °C下共氧化热解合成磁性生物炭的新方法。此外,应用磁性生物炭去除水溶液中的四环素和铜(II)。这种共氧化热解成功地将磁铁矿、零价铁和锰铁氧体浸渍到含有锰氧化物的碳基体中,温度为250 °C。同时,形成了多孔结构,伴随着丰富的氧基团。该磁性生物炭在35 ℃下对四环素和铜(II)的吸附量分别为720.7和267.9 mg/g,饱和磁化强度为21.79 emu/g。磁性生物炭在实际水基质中仍具有较高的吸附性能,显示出其在工业废水处理中的巨大潜力。对四环素的吸附主要是氢键/络合/π-π相互作用/孔填充,对铜(II)的吸附主要是阳离子交换/络合/静电相互作用/孔填充。高锰酸钾和高铁酸钾低温共氧化热解制备高吸附性磁性生物炭的成功,证明氧化磁化是一种很有前途的低能耗高性能磁性生物炭合成技术。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Science
工程技术-工程:化工
CiteScore
7.50
自引率
8.50%
发文量
1025
审稿时长
50 days
期刊介绍:
Chemical engineering enables the transformation of natural resources and energy into useful products for society. It draws on and applies natural sciences, mathematics and economics, and has developed fundamental engineering science that underpins the discipline.
Chemical Engineering Science (CES) has been publishing papers on the fundamentals of chemical engineering since 1951. CES is the platform where the most significant advances in the discipline have ever since been published. Chemical Engineering Science has accompanied and sustained chemical engineering through its development into the vibrant and broad scientific discipline it is today.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


