A melt-based reaction pathway for CO2 and CH4 conversion to syngas and carbon using liquid In–Sn
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Molten In–Sn has been recently reported as an effective catalyst for directly producing 2:1 H2:CO syngas and solid carbon from CO2 and CH4 in a single reaction, equivalent to the combined reactions of methane pyrolysis and dry reforming. In the present work, when reactants are alternately fed, mass balances indicate that CO2 reacts to form an [O] species that oxidizes CH4. Also, CH4 is converted to a [C] species that reduces CO2. The presence of accumulated [O] did not significantly affect the experimentally determined activation energies of 229 kJ/mol vs. 222 kJ/mol. We performed ab initio molecular dynamics (AIMD) simulations and density functional theory (DFT) calculations, to probe the dynamic structural evolution and to obtain atomistic insights into the behavior of the molten In–Sn alloy at the molecular level. This integrated approach also facilitated the evaluation of activation energy barriers associated with key reaction pathways. Simulations indicate that [O] are solvated; rapidly switching neighbors between Sn and In, which is unique to a molten catalyst. The presence of accumulated [C] significantly decreased the experimentally observed apparent CO2 activation energy from 154 kJ/mol to 75 kJ/mol, which is lower than the direct reaction between CO2 and solid graphite. This finding supports the simulations that indicate [C] formed in the melt is solvated, chemically distinct from solid carbon, and can play a critical role in enhancing catalytic performance. Large fluctuations in adsorbate binding energies were theoretically observed over time, suggesting the creation of transient sites that are fleetingly active, and facilitate the formation of solvated [O] and [C] intermediates unique to a molten catalyst surface.
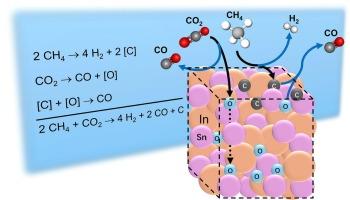
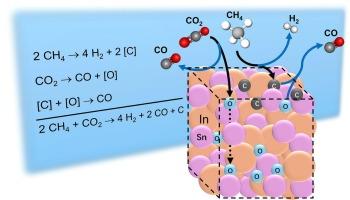
利用液态In-Sn将CO2和CH4转化为合成气和碳的熔融反应途径
熔融in - sn是一种有效的催化剂,可以直接由CO2和CH4在一个反应中生成2:1 H2:CO合成气和固体碳,相当于甲烷热解和干重整的联合反应。在目前的工作中,当反应物交替投料时,质量平衡表明CO2反应形成氧化CH4的[O]物质。同时,CH4转化为a [C]种,减少CO2。[O]的存在对229 kJ/mol和222 kJ/mol的活化能的影响不显著。通过从头算分子动力学(AIMD)模拟和密度泛函理论(DFT)计算,探讨了In-Sn合金的动态结构演变,并在分子水平上获得了对熔融In-Sn合金行为的原子性见解。这种综合方法还有助于评估与关键反应途径相关的活化能垒。模拟表明[O]被溶剂化;在Sn和In之间快速切换邻居,这是熔融催化剂所特有的。[C]的存在显著降低了实验观测到的CO2表观活化能,从154 kJ/mol降低到75 kJ/mol,低于CO2与固体石墨的直接反应。这一发现支持了模拟结果,表明熔体中形成的[C]是溶剂化的,在化学上与固体碳不同,可以在提高催化性能方面发挥关键作用。理论上,随着时间的推移,观察到吸附质结合能的大幅波动,表明瞬态位点的产生具有短暂的活性,并促进了熔融催化剂表面特有的溶剂化[O]和[C]中间体的形成。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


