Paternal exercise confers endurance capacity to offspring through sperm microRNAs
IF 30.9
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
Paternal exercise influences exercise capacity and metabolic health of offspring, but the underlying mechanisms remain poorly understood. We demonstrate that offspring sired by exercise-trained fathers display intrinsic exercise adaptations and improved metabolic parameters compared with those sired by sedentary fathers. Similarly, offspring born to transgenic mice with muscle-specific overexpression of peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α), a booster of mitochondrial function, exhibit improved endurance capacity and metabolic traits, even in the absence of the inherited PGC-1α transgene. Injecting sperm small RNAs from exercised fathers into normal zygotes recapitulates exercise-trained phenotypes in offspring at the behavioral, metabolic, and molecular levels. Mechanistically, exercise training and muscular PGC-1α overexpression remodel sperm microRNAs, which directly suppress nuclear receptor corepressor 1 (NCoR1), a functional antagonist of PGC-1α, in early embryos, thereby reprogramming transcriptional networks to promote mitochondrial biogenesis and oxidative metabolism. Overall, this study underscores a causal role for paternal PGC-1α, sperm microRNAs, and embryonic NCoR1 in transmitting exercise-induced phenotypes and metabolic adaptations to offspring.
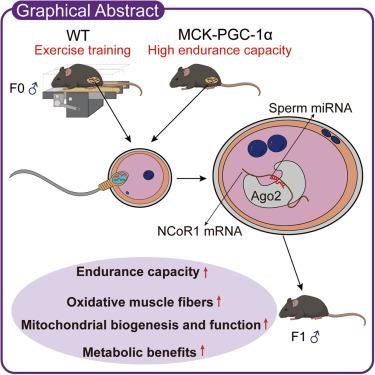
父亲的运动通过精子微rna赋予后代耐力
父亲的运动影响后代的运动能力和代谢健康,但潜在的机制尚不清楚。我们证明,与久坐不动的父亲所生的后代相比,运动训练的父亲所生的后代表现出内在的运动适应性和改善的代谢参数。同样,即使没有遗传的过氧化物酶体增殖物激活受体γ共激活因子-1α (PGC-1α)转基因小鼠的后代,其肌肉特异性过表达过氧化物酶体增殖物激活受体γ共激活因子-1α (PGC-1α),线粒体功能的助推器,表现出更好的耐力和代谢特征。将来自运动父亲的精子小rna注射到正常受精卵中,在行为、代谢和分子水平上再现了后代的运动训练表型。在机制上,运动训练和肌肉PGC-1α过表达重塑了精子microrna,这些microrna直接抑制早期胚胎中PGC-1α的功能性拮抗剂核受体协同抑制因子1 (NCoR1),从而重新编程转录网络,促进线粒体生物发生和氧化代谢。总的来说,本研究强调了父本PGC-1α、精子microrna和胚胎NCoR1在将运动诱导的表型和代谢适应传递给后代中的因果作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell metabolism
生物-内分泌学与代谢
CiteScore
48.60
自引率
1.40%
发文量
173
审稿时长
2.5 months
期刊介绍:
Cell Metabolism is a top research journal established in 2005 that focuses on publishing original and impactful papers in the field of metabolic research.It covers a wide range of topics including diabetes, obesity, cardiovascular biology, aging and stress responses, circadian biology, and many others.
Cell Metabolism aims to contribute to the advancement of metabolic research by providing a platform for the publication and dissemination of high-quality research and thought-provoking articles.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


