Identification of a novel compound containing benzyloxy terminal structure as a selective TYK2 inhibitor for the treatment of inflammatory diseases
IF 5.9
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
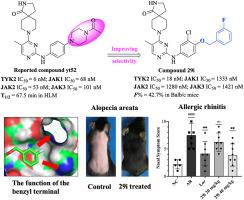
In this study, we described three series of N-phenylpyrimidin-2-amine derivatives as selective TYK2 inhibitors. Systematic exploration of the structure-activity relationship through the introduction of an O-linker and the flexible benzyl substituent based on the reported non-selective JAKs inhibitor yt52 led to the discovery of the optimized derivative compound 29i. Compound 29i showed a potency on TYK2 with an IC50 value of 18 nM and exhibited more than >70-fold selectivity over JAK1/2/3 isoforms. Kinase panel screening, WB assays, and human peripheral blood mononuclear cell assays further validated the selectivity of compound 29i. Compound 29i demonstrated pharmacokinetic properties with an oral bioavailability of 42.7 %. Moreover, in models of inflammatory disease (allergic rhinitis) and autoimmune disease (alopecia areata), compound 29i demonstrated comparable therapeutic effects to market drugs. Taken together, these findings establish that compound 29i is a selective TYK2 inhibitor with compelling potential for clinical development.
鉴定一种含有苯氧基末端结构的新型化合物,作为治疗炎性疾病的选择性TYK2抑制剂
在这项研究中,我们描述了三个系列的n-苯基嘧啶-2-胺衍生物作为选择性TYK2抑制剂。在已报道的非选择性JAKs抑制剂yt52的基础上,通过引入o -连接体和柔性苄基取代基,系统地探索了其构效关系,发现了优化的衍生物29i。化合物29i对TYK2的IC50值为18 nM,对JAK1/2/3亚型的选择性超过70倍。激酶组筛选、WB试验和人外周血单个核细胞试验进一步验证了化合物29i的选择性。化合物29i具有良好的药代动力学特性,口服生物利用度为42.7% %。此外,在炎症性疾病(变应性鼻炎)和自身免疫性疾病(斑秃)模型中,化合物29i显示出与市场上的药物相当的治疗效果。综上所述,这些发现表明化合物29i是一种具有临床开发潜力的选择性TYK2抑制剂。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


