Divergent Transformation of Diarylethanones via Anodic Oxidation and Electrochemical Baeyer–Villiger Oxidation for Scalable Synthesis of α-Acyloxyketones
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
A divergent electrochemical transformation of diarylethanones using molecular oxygen and electricity as green oxidants provides a sustainable and scalable strategy for synthesizing α-acyloxyketone compounds. Mechanistic studies reveal bromide-mediated generation of a benzyl radical cation intermediate, which undergoes divergent reaction pathways: (i) anodic oxidation to a benzyl cation or (ii) reaction with cathodically generated O2•– followed by Baeyer–Villiger oxidation to a carboxylate anion.
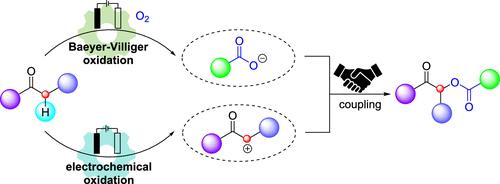
二芳基乙烷的阳极氧化和电化学Baeyer-Villiger氧化发散转化制备α-酰基氧酮。
利用分子氧和电作为绿色氧化剂对二乙基乙烷进行发散电化学转化,为合成α-酰基氧酮化合物提供了一种可持续的、可扩展的策略。机理研究揭示了溴介导的苯自由基阳离子中间体的生成,该中间体经历了不同的反应途径:(i)阳极氧化生成苯基阳离子或(ii)与阴极生成的O2•-反应,随后Baeyer-Villiger氧化生成羧酸阴离子。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


