Programmable delayed-release container system (PDRCS) for chronotherapeutic delivery: application in personalized treatment for Addison’s disease
IF 5.2
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
Chronopharmaceutical systems aim to synchronize drug release with the body’s biological rhythms to enhance therapeutic efficacy and minimize side effects. Current oral delivery technologies largely depend on coating technologies and pH-sensitive polymers, which are limited by significant inter- and intra-patient variability, as well as technical constraints in coating reproducibility. To address these challenges, we present a novel 3D-printed Programmable Delayed-Release Container System (PDRCS) for pH-independent, time-specific pulsatile drug delivery. Hydrocortisone was selected as the model drug to demonstrate chronotherapeutic targeting for primary adrenal insufficiency (Addison’s disease). The system consists of an ethyl cellulose shell manufactured via fused deposition modeling (FDM), encapsulating a layered core comprising a swelling hydrogel disc, a separating barrier, and an immediate-release hydrocortisone tablet. Upon immersion in dissolution media, water enters through engineered perforations, triggering the swelling disc to expand and build internal pressure until rupture of the shell occurs, resulting in drug release. By adjusting perforation diameters 1.0, 1.5, and 2.0 mm, lag times of 28, 20, and 12 h, respectively, were achieved. In vitro studies confirmed the system’s pH-independent behavior. Solid-state characterization (PXRD, FTIR, DSC, TGA) validated formulation stability, processing integrity, and revealing an increase in crystallinity after extrusion followed by reduction upon 3D printing. SEM imaging, rupture force analysis, and hydrogel swelling test were conducted to characterize the rupture behavior. This mechanically governed, rupture-based delivery platform enables customizable and reliable time-controlled oral drug administration, supporting personalized chronotherapeutic regimens.
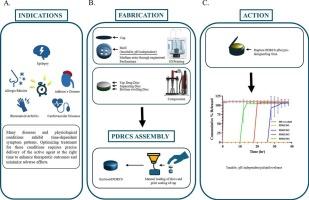
可编程延迟释放容器系统(PDRCS)用于时间治疗递送:在艾迪生病个性化治疗中的应用。
时间制药系统旨在使药物释放与人体的生物节律同步,以提高治疗效果并减少副作用。目前的口服给药技术在很大程度上依赖于涂层技术和ph敏感聚合物,这些技术受到患者之间和患者内部差异以及涂层可重复性的技术限制。为了解决这些挑战,我们提出了一种新型的3d打印可编程延迟释放容器系统(PDRCS),用于ph无关的,特定时间的脉冲药物输送。选择氢化可的松作为模型药物来证明原发性肾上腺功能不全(Addison's病)的时间治疗靶向性。该系统由通过熔融沉积建模(FDM)制造的乙基纤维素外壳组成,封装了由膨胀水凝胶盘、分离屏障和立即释放的氢化可的松片组成的层状核心。在溶解介质中浸泡后,水通过设计的穿孔进入,触发膨胀盘膨胀并形成内部压力,直到外壳破裂,导致药物释放。通过调整射孔直径1.0、1.5和2.0 mm,延迟时间分别为28、20和12 h。体外研究证实了该系统的ph无关行为。固态表征(PXRD, FTIR, DSC, TGA)验证了配方的稳定性,加工的完整性,并揭示了挤出后结晶度的增加和3D打印后的降低。通过扫描电镜成像、破裂力分析和水凝胶膨胀试验来表征其破裂行为。这种机械控制的、基于破裂的给药平台能够实现可定制和可靠的时间控制口服药物给药,支持个性化的时间治疗方案。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
10.70
自引率
8.60%
发文量
951
审稿时长
72 days
期刊介绍:
The International Journal of Pharmaceutics is the third most cited journal in the "Pharmacy & Pharmacology" category out of 366 journals, being the true home for pharmaceutical scientists concerned with the physical, chemical and biological properties of devices and delivery systems for drugs, vaccines and biologicals, including their design, manufacture and evaluation. This includes evaluation of the properties of drugs, excipients such as surfactants and polymers and novel materials. The journal has special sections on pharmaceutical nanotechnology and personalized medicines, and publishes research papers, reviews, commentaries and letters to the editor as well as special issues.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


