Bioactive scalarane-type sesterterpenoids from the Yongle Islands marine sponge Phyllospongia foliascens
IF 2.2
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
A further and systematic chemical investigation was carried out on the marine sponge Phyllospongia foliascens, collected from the Yongle Islands in the South China Sea, leading to the isolation of 17 scalarane-type sesterterpenoids, including five new compounds (1–5) and twelve known analogues (6–17). Their structures were determined using spectral analysis in conjunction with NMR/ECD calculations. Among the compounds, the pentacyclic sesterterpenoids 3 and 4 (featuring a 6/6/6/6/5 ring system) exhibited signal splitting in their 13C NMR spectra recorded in CDCl3. This challenge was resolved by stabilizing a single configuration through NMR solvent alteration and NMR experiment temperature variation, providing a reference strategy for similar issues. Cytotoxicity evaluation using the CCK-8 assay revealed that compound 3 was active against both K562 and U937 cell lines (IC50 = 9.00 μM and 8.45 μM, respectively), while compound 4 exhibited significant and selective cytotoxicity against K562 cells (IC50 = 5.69 μM).
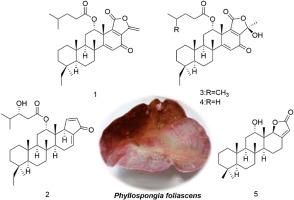
永乐岛海绵叶根海绵的生物活性角鲨烷型酯萜类化合物
对南海永乐群岛海绵体叶根海绵(Phyllospongia foliascens)进行了进一步系统的化学分析,分离得到17个角鲨烷型酯萜类化合物,包括5个新化合物(1-5)和12个已知类似物(6-17)。它们的结构是通过结合NMR/ECD计算的光谱分析来确定的。在CDCl3中记录的13C NMR谱中,五环酯萜类化合物3和4(具有6/6/6/6/ 6/5环体系)出现了信号分裂。通过核磁共振溶剂改变和核磁共振实验温度变化来稳定单一构型,解决了这一挑战,为类似问题提供了参考策略。CCK-8细胞毒性评价表明,化合物3对K562和U937细胞均有活性(IC50分别为9.00 μM和8.45 μM),而化合物4对K562细胞具有显著的选择性细胞毒性(IC50为5.69 μM)。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Tetrahedron
化学-有机化学
CiteScore
3.90
自引率
4.80%
发文量
439
审稿时长
34 days
期刊介绍:
Tetrahedron publishes full accounts of research having outstanding significance in the broad field of organic chemistry and its related disciplines, such as organic materials and bio-organic chemistry.
Regular papers in Tetrahedron are expected to represent detailed accounts of an original study having substantially greater scope and details than that found in a communication, as published in Tetrahedron Letters.
Tetrahedron also publishes thematic collections of papers as special issues and ''Reports'', commissioned in-depth reviews providing a comprehensive overview of a research area.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


