Investigating zinc-doped silicate-based trinary glass using computational chemistry: A study on interatomic interaction, dissolution behavior, and antibacterial efficiency
IF 3.5
3区 材料科学
Q1 MATERIALS SCIENCE, CERAMICS
引用次数: 0
Abstract
Molecular dynamics (MD) simulations were employed to investigate the structural and dissolution behavior of silicate-based Zn-doped glasses with a composition of 60SiO2-(40-x)CaO-xZnO (x = 1, 5, 10, 15, 20 mol%), labeled Zn1–Zn20. Using LAMMPS, the short- and medium-range order structures were analyzed. Short-range properties matched previous studies, validating the potential parameters, while bridging oxygens (BOs) and non-bridging oxygens (NBOs) remained unchanged, showing that ZnO substitution did not alter oxygen behavior. Zn20 had the highest density (2.82 g/cm3) versus 2.62 g/cm3 for Zn1, which was attributed to Zn’s higher molar mass. Network connectivity (NC) was slightly lower in Zn1 and Zn5 (2.71) than at higher Zn contents (2.73), suggesting lower solubility at higher ZnO contents. The clustering increased with ZnO as RCa-Ca increased from 1.05 (Zn1) to 1.31 (Zn20). ICP-AES after 72 h in SBF showed reduced Si release (30 ppm Zn1 vs. 12 ppm Zn20) and higher pH (7.45 ± 0.045 Zn1 vs. 7.590 ± 0.048 Zn20). Antibacterial tests against Escherichia coli revealed that Zn5 had the strongest effect (***p ˂ 0.001). Overall, unlike previous studies that focused only on experiments, this work combined MD simulations and experiments to identify Zn5 as the optimized composition, showing efficient solubility, dissolution, and antibacterial activity, making it promising for biomedical and tissue engineering.
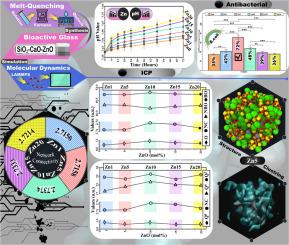
用计算化学方法研究锌掺杂硅酸盐基三元玻璃:原子间相互作用、溶解行为和抗菌效率的研究
采用分子动力学(MD)模拟研究了60SiO2-(40-x)CaO-xZnO (x = 1,5,10,15,20 mol%)标记为Zn1-Zn20的硅酸盐基掺锌玻璃的结构和溶解行为。利用LAMMPS分析了中短期订单结构。短程性质与先前的研究相匹配,验证了电位参数,而桥接氧(BOs)和非桥接氧(NBOs)保持不变,表明ZnO取代没有改变氧行为。Zn20的密度最高,为2.82 g/cm3,而Zn1的密度为2.62 g/cm3,这是由于Zn的摩尔质量更高。Zn1和Zn5的网络连通性(NC)(2.71)略低于锌含量较高时的(2.73),表明ZnO含量较高时溶解度较低。随着ZnO的增加,RCa-Ca由1.05 (Zn1)增加到1.31 (Zn20)。在SBF中浸泡72 h后,ICP-AES显示Si释放量减少(30 ppm Zn1比12 ppm Zn20), pH值升高(7.45±0.045 Zn1比7.590±0.048 Zn20)。对大肠杆菌的抑菌试验表明,Zn5的抑菌效果最强(***p小于0.001)。总的来说,与以往的研究只关注实验不同,本研究将MD模拟和实验相结合,确定了Zn5是优化的组合物,具有高效的溶解度、溶解性和抗菌活性,在生物医学和组织工程方面具有广阔的应用前景。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Non-crystalline Solids
工程技术-材料科学:硅酸盐
CiteScore
6.50
自引率
11.40%
发文量
576
审稿时长
35 days
期刊介绍:
The Journal of Non-Crystalline Solids publishes review articles, research papers, and Letters to the Editor on amorphous and glassy materials, including inorganic, organic, polymeric, hybrid and metallic systems. Papers on partially glassy materials, such as glass-ceramics and glass-matrix composites, and papers involving the liquid state are also included in so far as the properties of the liquid are relevant for the formation of the solid.
In all cases the papers must demonstrate both novelty and importance to the field, by way of significant advances in understanding or application of non-crystalline solids; in the case of Letters, a compelling case must also be made for expedited handling.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


