Pt dopants in ruthenium/iridium oxides promote catalytic activity in overall acidic water splitting
IF 8.9
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
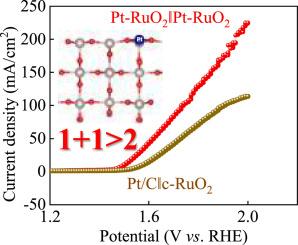
The commercialization of polymer electrolyte membrane water splitting technology significantly depends on the oxygen/hydrogen evolution reaction (OER/HER) electrocatalysts; customarily catalyzed by platinum (Pt) and ruthenium/iridium oxides (RuO2/IrO2). In this work, we have devised a novel strategy to improve the catalytic activities towards OER and HER catalysis via the decoration of RuO2 with Pt. Pt dopants in ruthenium oxides (Pt-RuO2) create more oxygen vacancies inducing a weaker interaction between active site and oxygen reaction intermediates, evidenced by downshifted d band center and increment in eg orbital filling of Ru atom; thereby, the acidic OER performance of Pt-RuO2 is enhanced by 3.5-fold than commercial RuO2 by mean of turnover frequency at 1.6 V vs. RHE. Moreover, Pt-RuO2 exhibits a similar HER performance to commercial Pt/C. The potential for overall water splitting is decreased by 0.18 V at 100 mA/cm2; besides, an excellent stability is also recorded after the incorporation of Pt dopants. The Δεd-p value of Pt-RuO2 was 1.76 eV, which is lower than the counterpart of RuO2, suggesting easy electron transition between d and p orbitals, suppressing the over-oxidation of RuO2; thereby, a higher stability is achieved for Pt-RuO2. The invitation of Pt dopants to boost catalytic activity and stability has also been extended to IrO2.
钌/铱氧化物中铂的掺杂促进了整体酸性水裂解的催化活性
聚合物电解质膜水分解技术的商业化在很大程度上取决于氧/氢析出反应(OER/HER)电催化剂;通常由铂(Pt)和钌/铱氧化物(RuO2/IrO2)催化。在本研究中,我们设计了一种新的策略,通过用Pt修饰RuO2来提高对OER和HER的催化活性。钌氧化物(Pt-RuO2)中的Pt掺杂物产生更多的氧空位,导致活性位点与氧反应中间体之间的相互作用减弱,证明了d能带中心的下降和Ru原子eg轨道填充的增加;因此,Pt-RuO2的酸性OER性能比商用RuO2提高了3.5倍,平均周转频率为1.6 V vs. RHE。此外,Pt- ruo2表现出与商用Pt/C相似的HER性能。在100 mA/cm2时,总水分解电位降低了0.18 V;此外,加入Pt掺杂剂后,还记录了良好的稳定性。Pt-RuO2的Δεd-p值为1.76 eV,低于RuO2,说明Pt-RuO2易于在d轨道和p轨道之间发生电子跃迁,抑制了RuO2的过度氧化;因此,Pt-RuO2具有更高的稳定性。Pt掺杂剂提高催化活性和稳定性的邀请也扩展到IrO2。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chinese Chemical Letters
化学-化学综合
CiteScore
14.10
自引率
15.40%
发文量
8969
审稿时长
1.6 months
期刊介绍:
Chinese Chemical Letters (CCL) (ISSN 1001-8417) was founded in July 1990. The journal publishes preliminary accounts in the whole field of chemistry, including inorganic chemistry, organic chemistry, analytical chemistry, physical chemistry, polymer chemistry, applied chemistry, etc.Chinese Chemical Letters does not accept articles previously published or scheduled to be published. To verify originality, your article may be checked by the originality detection service CrossCheck.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


