Isolation and characterization of peroxidase from potato leaves Solanum tuberosum: Application in glucose diagnostic kit
IF 2.8
Q3 Biochemistry, Genetics and Molecular Biology
Journal of Genetic Engineering and Biotechnology
Pub Date : 2025-09-30
DOI:10.1016/j.jgeb.2025.100584
引用次数: 0
Abstract
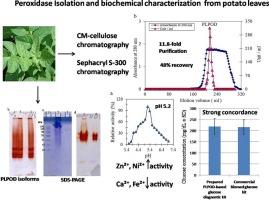
Peroxidases play a pivotal role in many medical applications such as diagnostic kits and ELISA assays. This study reports the purification and biochemical characterization of peroxidase from potato leaves (PLPOD) and its application in the formulation of a glucose diagnostic kit. PLPOD was purified through CM-cellulose ion-exchange and Sephacryl S-300 gel filtration chromatography, achieving an 11.8-fold purification with 48 % recovery and a final specific activity of 705.7 U/mg. Native PAGE and activity staining confirmed the enzyme’s purity and homogeneity. PLPOD molecular weight was estimated from gel filtration column as 64 kDa, but on SDS gel, there were three PLPOD isoforms of approximated molecular weights ranging from ∼ 40–60 kDa. PLPOD exhibited optimal activity at pH 5.2, with Zn2+ and Ni2+ enhancing activity, while Ca2+ and Fe2+ inhibited it. Inhibitor analysis confirmed the heme-dependent nature of the enzyme. The Km values for guaiacol and H2O2 were 0.067 mM and 40 mM, respectively, consistent with typical plant peroxidases. A glucose diagnostic kit developed using PLPOD showed strong concordance with a commercial glucose kit when tested on normal and diabetic serum samples demonstrating its clinical applicability. These findings suggest that PLPOD is a viable cost-effective for use in diagnostic assays and kits.
马铃薯叶片中过氧化物酶的分离与鉴定:在葡萄糖诊断试剂盒中的应用
过氧化物酶在许多医学应用中发挥着关键作用,如诊断试剂盒和酶联免疫吸附试验。本研究报道了马铃薯叶片过氧化物酶(PLPOD)的纯化、生化特性及其在葡萄糖诊断试剂盒中的应用。通过cm -纤维素离子交换和sepphacryl S-300凝胶过滤层析纯化PLPOD,纯化率为11.8倍,回收率为48%,最终比活性为705.7 U/mg。天然PAGE和活性染色证实了酶的纯度和均匀性。从凝胶过滤柱上估计PLPOD的分子量为64 kDa,但在SDS凝胶上,PLPOD的三种异构体的近似分子量在~ 40-60 kDa之间。PLPOD在pH 5.2时活性最佳,Zn2+和Ni2+增强了PLPOD活性,Ca2+和Fe2+抑制了PLPOD活性。抑制剂分析证实了该酶的血红素依赖性。愈创木酚和H2O2的Km值分别为0.067 mM和40 mM,与典型植物过氧化物酶一致。使用PLPOD开发的葡萄糖诊断试剂盒在对正常和糖尿病血清样本进行测试时显示出与商业葡萄糖试剂盒的强一致性,证明了其临床适用性。这些发现表明,PLPOD在诊断分析和试剂盒中是一种具有成本效益的可行方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Genetic Engineering and Biotechnology
Biochemistry, Genetics and Molecular Biology-Biotechnology
CiteScore
5.70
自引率
5.70%
发文量
159
审稿时长
16 weeks
期刊介绍:
Journal of genetic engineering and biotechnology is devoted to rapid publication of full-length research papers that leads to significant contribution in advancing knowledge in genetic engineering and biotechnology and provide novel perspectives in this research area. JGEB includes all major themes related to genetic engineering and recombinant DNA. The area of interest of JGEB includes but not restricted to: •Plant genetics •Animal genetics •Bacterial enzymes •Agricultural Biotechnology, •Biochemistry, •Biophysics, •Bioinformatics, •Environmental Biotechnology, •Industrial Biotechnology, •Microbial biotechnology, •Medical Biotechnology, •Bioenergy, Biosafety, •Biosecurity, •Bioethics, •GMOS, •Genomic, •Proteomic JGEB accepts
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


