Royal jelly acid inhibits NF-κB signaling by regulating H3 histone lactylation to alleviate IgE-mediated mast cell activation and allergic inflammation
IF 8.3
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
Background
Mast cells (MCs) mediate high-affinity IgE Fc receptor (FcεRI)-mediated allergic reactions. Royal jelly acid (RJA), the major lipid constituent of royal jelly, has anti-inflammatory, anti-tumor, antibacterial, and immunomodulatory properties.
Purpose
This study aimed to investigate how RJA affects IgE-mediated MC activation and to elucidate mechanisms underlying anti-allergic effects.
Methods
Mouse bone marrow-derived mast cells (BMMCs) and rat basophilic leukemia cells-2H3 (RBLs) were stimulated with IgE-antigen (Ag). β-hexosaminidase (β‑hex) and histamine (HA) release, inflammatory cytokines expression, and cellular morphology changes were examined in stimulated cells. Transcriptome changes associated with RJA-treated activated-MCs were analyzed. Network pharmacology, gene expression, and western blot analyses were employed to explore the relationship between H3 lactylation and anti-allergic effects of RJA on MC. In vivo studies were conducted in IgE-mediated passive cutaneous anaphylaxis (PCA), ovalbumin (OVA)-induced active systemic anaphylaxis (ASA), and HA-induced passive systemic anaphylaxis (PSA) mouse models.
Results
RJA inhibited IgE-dependent MC activation as evidenced by reduced β‑hex and HA release. RJA inhibited MAPK and NF-κB signaling, down-regulated RELA and NFκB1 transcripts, and decreased NF-κB reporter activity. RJA inhibited cellular H3 lactylation and H3K9 lactylation (H3K9la), leading to reduced histone lactyllysine enrichment in RELA or NFκB1 promoter loci. RJA alleviated allergic symptoms in PCA and ASA mice by intraperitoneal injection or oral administration, but did not affect HA-induced hypothermia.
Conclusion
RJA inhibits IgE-dependent MC activation by reducing cellular H3K9la and suppressing NF-κB signaling via reduced histone lactyllysine enrichment in RELA or NFκB1 promoter sites. RJA may support MC inhibition in the prevention and treatment of allergic diseases.
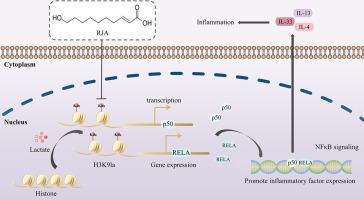
蜂王浆酸通过调节H3组蛋白乳酸化抑制NF-κB信号,减轻ige介导的肥大细胞活化和过敏性炎症
肥大细胞(MCs)介导高亲和力IgE Fc受体(FcεRI)介导的过敏反应。蜂王浆酸(RJA)是蜂王浆的主要脂质成分,具有抗炎、抗肿瘤、抗菌和免疫调节等特性。目的研究RJA如何影响ige介导的MC激活,并阐明其抗过敏作用的机制。方法用ige抗原(Ag)刺激小鼠骨髓源性肥大细胞(BMMCs)和大鼠嗜碱性白血病细胞- 2h3 (RBLs)。在受刺激的细胞中检测β-己糖氨酸酶(β -hex)和组胺(HA)释放、炎症因子表达和细胞形态学变化。分析rja处理的活化mcs的转录组变化。采用网络药理学、基因表达、western blot等方法探讨RJA对MC的抗过敏作用与H3乳酸化的关系。在ige介导的被动皮肤过敏反应(PCA)、卵清蛋白(OVA)诱导的主动全身过敏反应(ASA)和ha诱导的被动全身过敏反应(PSA)小鼠模型中进行了体内研究。结果rja抑制了ige依赖性MC的激活,β - hex和HA的释放减少。RJA抑制MAPK和NF-κB信号,下调RELA和NF-κ b1转录本,降低NF-κB报告基因活性。RJA抑制细胞H3和H3K9乙酰化(H3K9la),导致RELA或NFκB1启动子位点组蛋白乙酰赖氨酸富集减少。RJA可通过腹腔注射或口服给药减轻PCA和ASA小鼠的过敏症状,但对ha诱导的低温无影响。结论rja通过降低RELA或NFκB1启动子位点组蛋白乙酰赖氨酸的富集,降低细胞H3K9la,抑制NF-κB信号传导,从而抑制ige依赖性MC的激活。RJA可能支持MC抑制预防和治疗过敏性疾病。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Phytomedicine
医学-药学
CiteScore
10.30
自引率
5.10%
发文量
670
审稿时长
91 days
期刊介绍:
Phytomedicine is a therapy-oriented journal that publishes innovative studies on the efficacy, safety, quality, and mechanisms of action of specified plant extracts, phytopharmaceuticals, and their isolated constituents. This includes clinical, pharmacological, pharmacokinetic, and toxicological studies of herbal medicinal products, preparations, and purified compounds with defined and consistent quality, ensuring reproducible pharmacological activity. Founded in 1994, Phytomedicine aims to focus and stimulate research in this field and establish internationally accepted scientific standards for pharmacological studies, proof of clinical efficacy, and safety of phytomedicines.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


