Fenbendazole–Amino Acid Derivatives as Novel Anthelmintic Candidates: Synthesis, Activity Against Haemonchus contortus, and In Silico Profiling.
IF 5.9
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
We report the synthesis and biological evaluation of fifteen fenbendazole–amino acid derivatives from a δ-valerolactam-based scaffold. These compounds were designed to enhance anthelmintic efficacy while reducing mammalian cytotoxicity. Their activity was assessed against Haemonchus contortus at both the exsheathed third-stage larval (xL3) and adult stages. Several derivatives showed early anthelmintic activity (24 h) and low cytotoxicity towards murine macrophages. In silico analysis indicated a correlation between MLOGP–TPSA profiles and biological performance, suggesting improved cuticular diffusion. Compared with albendazole and fenbendazole, active compounds exhibited lower toxicity and occupied distinct regions in the physicochemical property space. These results support the potential of these new compounds as selective scaffolds for developing next-generation anthelmintics.
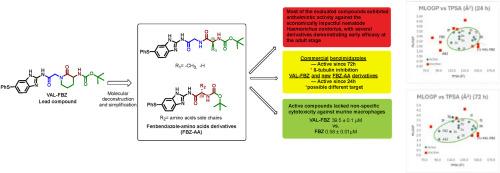
芬苯达唑-氨基酸衍生物作为新的候选驱虫药:合成、抗弯曲血蜱活性和硅谱分析。
我们报道了以δ-戊内酰胺为基础的支架合成了15种苯并达唑氨基酸衍生物并进行了生物学评价。这些化合物旨在增强驱虫药的功效,同时降低哺乳动物的细胞毒性。在脱毛后的第三期幼虫(xL3)和成虫期对弯曲血蜱的活性进行了评估。几种衍生物对小鼠巨噬细胞具有早期驱虫活性(24小时)和低细胞毒性。硅分析表明MLOGP-TPSA谱与生物性能之间存在相关性,表明表皮扩散得到改善。与阿苯达唑和芬苯达唑相比,活性化合物具有较低的毒性,在理化性质空间中占据不同的区域。这些结果支持了这些新化合物作为开发下一代驱虫药的选择性支架的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


