Novel PROTACs targeting tissue transglutaminase (TG2) suppress tumorigenicity of ovarian cancer cells
IF 5.9
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
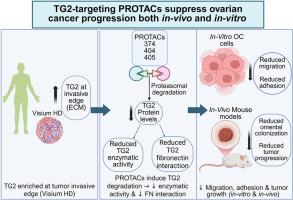
Tissue transglutaminase (TG2), a multifunctional enzyme involved in protein crosslinking through transamidation, fibronectin–integrin interactions and GTP hydrolysis, is upregulated in cancer. Due to its diverse functions, TG2 has been a challenging therapeutic target. Here, we investigate the use of PROteolysis TArgeting Chimeras (PROTACs) to degrade TG2 and inhibit its tumor-promoting functions in ovarian cancer models. We describe a novel family of VHL based PROTACs using a ligand that binds to the TG2 fibronectin interacting domain and a thiol ether PEG linker. Three structurally related PROTACs—P374, P404, and P405—induced significant proteasome dependent TG2 degradation at 24 hours (p < 0.05), with stable effects at 48 hours. These compounds also potently inhibited cell adhesion and migration (p < 0.005), outside-in signaling, and blocked TG2 enzymatic activity (p < 0.001). An unbiased evaluation using reverse phase protein array of P374-treated cells revealed 136 differentially expressed proteins, including protein networks related to cell adhesion and involved in extracellular matrix (ECM) interactions. P374 and P405 reduced omental colonization in vivo and P374 inhibited intraperitoneal tumor dissemination and growth. Visium HD based spatial profiling of human ovarian tumors identified TG2 as a highly enriched protein at the tumors invasive edge and the interface with the ECM. Together, our findings put forward novel TG2-targeting PROTACs which effectively degrade TG2, impair its functions, and block in-vivo tumor dissemination. These results highlight the potential development of TG2 degraders towards therapeutic targeting in ovarian cancer.
靶向组织转谷氨酰胺酶(TG2)的新型PROTACs抑制卵巢癌细胞的致瘤性
组织转谷氨酰胺酶(TG2)是一种多功能酶,参与通过转氨化、纤维连接蛋白-整合素相互作用和GTP水解进行蛋白质交联,在癌症中上调。由于其多种功能,TG2一直是一个具有挑战性的治疗靶点。在此,我们研究了在卵巢癌模型中使用靶向嵌合体(PROteolysis TArgeting Chimeras, PROTACs)降解TG2并抑制其促肿瘤功能。我们描述了一个新的基于VHL的PROTACs家族,使用一个结合TG2纤维连接蛋白相互作用域的配体和一个硫醚PEG连接体。三种结构相关的protacs——p374、P404和p405在24小时内诱导了蛋白酶体依赖性的TG2降解(p < 0.05),并在48小时内作用稳定。这些化合物还能有效抑制细胞粘附和迁移(p < 0.005)、外向内信号传导,并阻断TG2酶活性(p < 0.001)。使用p374处理的细胞的反相蛋白阵列进行无偏评价,发现136种差异表达蛋白,包括与细胞粘附相关的蛋白网络和参与细胞外基质(ECM)相互作用的蛋白网络。P374和P405减少了体内大网膜定植,P374抑制了腹膜内肿瘤的播散和生长。基于Visium HD的人卵巢肿瘤空间分析发现TG2是肿瘤侵袭边缘和ECM界面高度富集的蛋白。总之,我们的研究结果提出了新的靶向TG2的PROTACs,可以有效地降解TG2,破坏其功能,并阻断肿瘤在体内的传播。这些结果突出了TG2降解物在卵巢癌靶向治疗方面的潜在发展。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


