Preparation of iron-containing exfoliated graphite from graphite intercalation compounds with iron chloride treated by liquid ammonia and alkylamines
IF 4.9
3区 材料科学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Composites of exfoliated graphite (EG) with metals are promising magnetic sorbents for oil sorption and recovery. This work presents a method for preparation of iron-containing EG from graphite intercalation compound (GIC) with iron chloride and ammonia/amines complexes. GICs with FeCl3 were treated by liquid ammonia, methylamine and ethylamine, followed by rapid heat treatment in a nitrogen atmosphere. Thermal decomposition of ammonia-treated adduct resulted in the formation of metallic α-Fe on the EG surface, while methylamine-treated adduct produced a mixture of α-Fe and iron carbide Fe3C. The structure of both GIC and EG were investigated by XRD analysis and Mössbauer spectroscopy. Ammonia and methylamine react with iron chloride and form the complex compounds within the graphite matrix, whereas ethylamine shows weak reaction with GIC. The presence of alkyl groups in the amines influences both the composition of the iron-containing phase and the EG surface morphology. Iron interacts with carbon to form a γ-(Fe,C) alloy and iron carbide Fe3C and amorphous carbon coats the EG surface leading to increasing hydrophilicity. The resulting EG/Fe composites exhibited high saturation magnetization (up to 50 emu/g) and exceptional hydrocarbon sorption capacity (up to 30 g/g), demonstrating their potential as efficient magnetic sorbents for oil remediation.
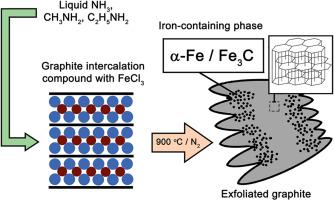
用液态氨和烷基胺处理氯化铁,用石墨插层化合物制备含铁剥落石墨
剥落石墨与金属复合材料是一种很有前途的磁性吸油剂。本文提出了一种以氯化铁和氨/胺配合物为原料,用石墨插层化合物(GIC)制备含铁EG的方法。采用液氨、甲胺和乙胺处理含FeCl3的GICs,然后在氮气气氛中快速热处理。氨处理的加合物在EG表面热分解生成金属α-Fe,而甲胺处理的加合物生成α-Fe和碳化铁Fe3C的混合物。通过XRD分析和Mössbauer光谱分析对GIC和EG的结构进行了研究。氨和甲胺与氯化铁反应在石墨基体内形成复合化合物,而乙胺与GIC反应弱。胺中烷基的存在影响了含铁相的组成和EG的表面形貌。铁与碳相互作用形成γ-(Fe,C)合金,铁碳化物Fe3C和无定形碳包覆在EG表面,提高亲水性。所得EG/Fe复合材料具有高饱和磁化强度(高达50 emu/g)和优异的碳氢化合物吸附能力(高达30 g/g),显示了其作为高效磁性吸附剂用于石油修复的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.80
自引率
2.50%
发文量
605
审稿时长
40 days
期刊介绍:
The Journal of Physics and Chemistry of Solids is a well-established international medium for publication of archival research in condensed matter and materials sciences. Areas of interest broadly include experimental and theoretical research on electronic, magnetic, spectroscopic and structural properties as well as the statistical mechanics and thermodynamics of materials. The focus is on gaining physical and chemical insight into the properties and potential applications of condensed matter systems.
Within the broad scope of the journal, beyond regular contributions, the editors have identified submissions in the following areas of physics and chemistry of solids to be of special current interest to the journal:
Low-dimensional systems
Exotic states of quantum electron matter including topological phases
Energy conversion and storage
Interfaces, nanoparticles and catalysts.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


