Oxygen driven defect and spin engineering in Ba0.5Sr0.5Co0.8Fe0.2O3–δ at high temperatures
IF 5.7
3区 材料科学
Q2 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
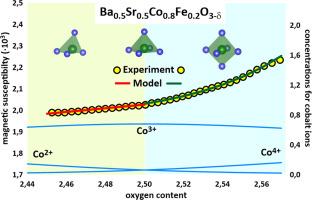
Non-stiochiometric Ba0.5Sr0.5Co0.8Fe0.2O3–δ oxide and its aluminum doped derivative were synthesized via combustion of organometallic precursors. The oxygen content was measured with temperature/oxygen partial pressure by thermogravimetry and coulometric titration techniques. A consistent defect formation model was developed for the first time. Aluminum doping was shown to reduce oxygen content and promote cobalt reduction. The model incorporates the coexistence of energetically non-equivalent oxygen sites, which were explicitly utilized in simulations. Functional dependencies of equilibrium defect concentrations, derived directly from the proposed defect structure, enable deep analysis of spin distribution over the 3d cations. Analysis of in-situ temperature dependent magnetic susceptibility measurements directly linked spin configurations of Co and Fe ions to their local environment. The validated model provides a fundamental basis for the rational design of functional cobaltite perovskites for high-temperature redox and ion transport applications and offers a tool for interpreting future transport property studies and thermal expansion behavior.
高温下ba0.5 sr0.5 co0.8 fe0.3 2o3 -δ的氧驱动缺陷及自旋工程
通过燃烧有机金属前驱体合成了非化学计量的ba0.5 sr0.5 co0.8 fe0.3 2o3 -δ氧化物及其铝掺杂衍生物。用热重法和库仑滴定法分别用温度/氧分压法测定氧含量。首次建立了一致的缺陷形成模型。铝掺杂可以降低氧含量,促进钴的还原。该模型包含了能量非等效氧位点的共存,这在模拟中得到了明确的利用。平衡缺陷浓度的功能依赖关系,直接来源于所提出的缺陷结构,可以深入分析三维阳离子的自旋分布。原位温度相关磁化率测量的分析将Co和Fe离子的自旋构型与其局部环境直接联系起来。验证的模型为合理设计用于高温氧化还原和离子传输的功能钴酸钙钛矿提供了基础,并为解释未来的传输性质研究和热膨胀行为提供了工具。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Materials Research Bulletin
工程技术-材料科学:综合
CiteScore
9.80
自引率
5.60%
发文量
372
审稿时长
42 days
期刊介绍:
Materials Research Bulletin is an international journal reporting high-impact research on processing-structure-property relationships in functional materials and nanomaterials with interesting electronic, magnetic, optical, thermal, mechanical or catalytic properties. Papers purely on thermodynamics or theoretical calculations (e.g., density functional theory) do not fall within the scope of the journal unless they also demonstrate a clear link to physical properties. Topics covered include functional materials (e.g., dielectrics, pyroelectrics, piezoelectrics, ferroelectrics, relaxors, thermoelectrics, etc.); electrochemistry and solid-state ionics (e.g., photovoltaics, batteries, sensors, and fuel cells); nanomaterials, graphene, and nanocomposites; luminescence and photocatalysis; crystal-structure and defect-structure analysis; novel electronics; non-crystalline solids; flexible electronics; protein-material interactions; and polymeric ion-exchange membranes.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


