Green voltammetric determination of the fungicide Fluopyram using a single-use pencil graphite electrode: Application to juice and urine samples
IF 4
1区 农林科学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Fluopyram, a broad-spectrum fungicide extensively used in agricultural systems to protect crops against a wide range of fungal pathogens, has raised increasing concern due to its potential persistence and accumulation in food and biological systems. Therefore, the development of simple, cost-effective, and highly sensitive detection strategies for monitoring Fluopyram residues is of considerable importance. In this study, we systematically explored the electrochemical properties and detection capability of Fluopyram using cyclic voltammetry (CV) and square-wave voltammetry (SWV) with a single-use pencil graphite electrode serving as the disposable working electrode. The CV measurements demonstrated that Fluopyram undergoes an irreversible oxidation process, yielding a well-defined anodic peak at approximately +1.05 V in a 0.04 M Britton–Robinson buffer solution at pH 4.0, which reflects a diffusion-controlled electron transfer mechanism. Quantification studies carried out with SWV revealed an excellent linear response over a wide concentration range from 0.04 to 1.23 μg/mL (R2 > 0.99). Notably, the method achieved a very low limit of detection of 0.009 μg/mL, underscoring the superior sensitivity of the proposed electroanalytical approach compared to many conventional methods. To evaluate its real-world applicability, the method was successfully applied to spiked urine and orange juice samples, where satisfactory recovery values confirmed its robustness in complex matrices. The electrochemical results were further validated by comparison with spectrophotometric measurements, demonstrating good consistency between the two techniques. Overall, this work introduces a disposable, rapid, and reliable electroanalytical platform for Fluopyram determination, offering promising potential for environmental monitoring, food safety assessment, and clinical applications.
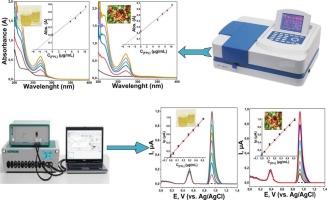
使用一次性铅笔石墨电极对杀菌剂Fluopyram的绿色伏安测定:应用于果汁和尿液样品
氟吡喃(Fluopyram)是一种广谱杀菌剂,广泛用于农业系统,以保护作物免受各种真菌病原体的侵害,由于其在食物和生物系统中的潜在持久性和积累性,引起了越来越多的关注。因此,开发一种简单、经济、高灵敏度的检测策略来监测氟吡喃残留是相当重要的。本研究以一次性铅笔石墨电极为工作电极,采用循环伏安法(CV)和方波伏安法(SWV)系统地探讨了Fluopyram的电化学性能和检测能力。CV测量表明,Fluopyram经历了一个不可逆的氧化过程,在pH为4.0的0.04 M briton - robinson缓冲溶液中,在大约+1.05 V处产生了一个明确的阳极峰,这反映了扩散控制的电子转移机制。用SWV进行的定量研究显示,在0.04 ~ 1.23 μg/mL的宽浓度范围内具有良好的线性响应(R2 > 0.99)。值得注意的是,该方法的检出限很低,仅为0.009 μg/mL,与许多传统方法相比,该方法具有更高的灵敏度。为了评估其在现实世界中的适用性,该方法成功地应用于加标尿液和橙汁样品,其中令人满意的回收率证实了其在复杂矩阵中的稳健性。通过与分光光度法测定结果的比较,进一步验证了电化学结果,表明两种方法具有良好的一致性。总之,这项工作介绍了一个一次性、快速、可靠的电分析平台,用于氟吡喃测定,为环境监测、食品安全评估和临床应用提供了广阔的前景。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.00
自引率
8.50%
发文量
238
审稿时长
4.2 months
期刊介绍:
Pesticide Biochemistry and Physiology publishes original scientific articles pertaining to the mode of action of plant protection agents such as insecticides, fungicides, herbicides, and similar compounds, including nonlethal pest control agents, biosynthesis of pheromones, hormones, and plant resistance agents. Manuscripts may include a biochemical, physiological, or molecular study for an understanding of comparative toxicology or selective toxicity of both target and nontarget organisms. Particular interest will be given to studies on the molecular biology of pest control, toxicology, and pesticide resistance.
Research Areas Emphasized Include the Biochemistry and Physiology of:
• Comparative toxicity
• Mode of action
• Pathophysiology
• Plant growth regulators
• Resistance
• Other effects of pesticides on both parasites and hosts.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


