Transformation mechanism of Fe impurities during Fe removal from vein quartz via low-temperature sulfuric acid roasting
IF 5
2区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
Removing Fe impurities is the key to purifying vein quartz for the production of high-purity quartz sand. However, conventional Fe removal methods suffer from HF contamination and operational complexity, hindering clean and efficient quartz purification. This study investigated the Fe removal efficiency and transformation mechanisms of Fe impurities in quartz using a low-temperature H2SO4 roasting followed by water leaching approach. Results indicated that roasting temperature serves as the predominant factor governing Fe speciation evolution during the H2SO4 roasting. When the roasting temperature was below 523 K, iron oxides impurities reacted with H2SO4 to form water-soluble Fe2(SO4)3. However, increasing the temperature above 523 K resulted in the decomposition of Fe2(SO4)3 into sparingly soluble Fe12O3(SO4)15. Thermal motion of H2SO4 facilitated the enrichment of the product Fe2(SO4)3 on the surface of quartz during the roasting process. Under conditions of roasting at 523 K and water leaching at 353 K, the content of Fe decreased from 980 μg·g−1 to 12.51 μg·g−1, thereby achieving a removal rate of up to 98.72 %. This study provides a clean and efficient method for preparing high-purity quartz sand using vein quartz.
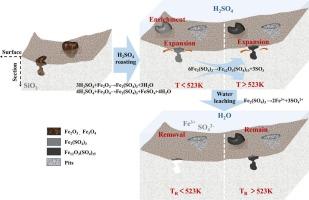
低温硫酸焙烧脱脉石英铁过程中铁杂质的转化机理
去除铁杂质是提纯脉石英生产高纯石英砂的关键。然而,传统的除铁方法受HF污染和操作复杂性的影响,阻碍了清洁高效的石英提纯。研究了低温硫酸焙烧-水浸法对石英中铁杂质的脱除效果及转化机理。结果表明,焙烧温度是控制H2SO4焙烧过程中铁形态演化的主要因素。当焙烧温度低于523 K时,氧化铁杂质与H2SO4反应生成水溶性Fe2(SO4)3。当温度升高到523 K以上时,Fe2(SO4)3分解成易溶的Fe12O3(SO4)15。在焙烧过程中,H2SO4的热运动促进了产物Fe2(SO4)3在石英表面的富集。在523 K焙烧、353 K水浸条件下,铁的含量由980 μg·g−1降至12.51 μg·g−1,去除率达98.72%。本研究为利用脉状石英制备高纯石英砂提供了一种清洁、高效的方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Minerals Engineering
工程技术-工程:化工
CiteScore
8.70
自引率
18.80%
发文量
519
审稿时长
81 days
期刊介绍:
The purpose of the journal is to provide for the rapid publication of topical papers featuring the latest developments in the allied fields of mineral processing and extractive metallurgy. Its wide ranging coverage of research and practical (operating) topics includes physical separation methods, such as comminution, flotation concentration and dewatering, chemical methods such as bio-, hydro-, and electro-metallurgy, analytical techniques, process control, simulation and instrumentation, and mineralogical aspects of processing. Environmental issues, particularly those pertaining to sustainable development, will also be strongly covered.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


