Preprocedural CT and ECG Markers for Predicting Post-TAVR Pacemaker Requirement in High-Risk Patients
IF 2.8
Q3 CARDIAC & CARDIOVASCULAR SYSTEMS
引用次数: 0
Abstract
Background
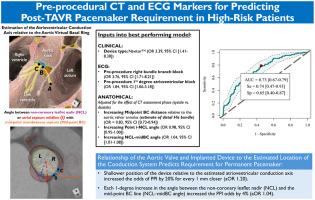
Need for permanent pacemaker implantation (PPI) following transcatheter aortic valve replacement (TAVR) remains a common complication. We aimed to assess computed tomography (CT)-based anatomical and electrocardiogram (ECG)-based parameters in a predictive model for PPI following TAVR.
Methods
We assessed CT-based parameters, including the predicted course of the conduction axis from atrioventricular node to left bundle branch origin relative to the aortic virtual basal ring. Electrophysiological variables were combined in assessing a model to predict post-TAVR PPI.
Results
Among 433 patients (mean age 82.0 [9.0] years, 54.0% female), 90 (21.0%) required PPI. Multiple binary logistic modeling demonstrated a shallower position of the membranous septum inferior margin midpoint increased the odds of PPI by 20% for every 1 mm (adjusted odds ratio [aOR]: 1.20) adjusted for the CT assessment phase. Increasing aortic root rotational angle associated with lower PPI odds (odds ratio [OR]: 0.98; 95% CI [0.95-1.00]), while an angle between the membranous septum midpoint and noncoronary leaflet nadir associated with increased PPI odds (OR: 1.04; 95% CI [1.01-1.08]). Preprocedural right bundle branch block and first-degree atrioventricular block associated with increased odds for PPI (OR: 3.76; 95% CI [1.71-8.21]; and OR: 1.84; 95% CI [1.06-3.18], respectively). The model had an area under the curve of 0.73 (95% CI [0.67-0.79]), sensitivity of 0.74 (95% CI [0.47-0.93]), and specificity of 0.65 (95% CI [0.40-0.87]) for predicting PPI requirement.
Conclusions
A predictive model for determining the risk of PPI following TAVR is reported, combining comprehensive conduction-specific anatomical measurements relative to the aortic root and electrical measurements with clinical parameters. This model requires prospective application to understand its performance in the real-world.
术前CT和ECG指标预测高危患者tavr术后起搏器需求
背景:经导管主动脉瓣置换术(TAVR)后需要永久性起搏器植入(PPI)仍然是一个常见的并发症。我们的目的是评估基于计算机断层扫描(CT)的解剖和基于心电图(ECG)的参数在TAVR后PPI预测模型中的应用。方法我们评估了基于ct的参数,包括相对于主动脉虚拟基环从房室结到左束支起源的传导轴的预测路线。结合电生理变量评估预测tavr后PPI的模型。结果433例患者(平均年龄82.0[9.0]岁,女性54.0%)中,90例(21.0%)需要使用PPI。多重二元logistic模型显示,隔膜下缘中点位置越浅,每1 mm PPI的发生率增加20%(校正比值比[aOR]: 1.20)。主动脉根部旋转角度增加与PPI风险降低相关(比值比[OR]: 0.98; 95% CI[0.95-1.00]),而隔膜中点与非冠状动脉小叶最低点之间的角度增加与PPI风险增加相关(OR: 1.04; 95% CI[1.01-1.08])。术前右束分支阻滞和一级房室传导阻滞与PPI发生率增加相关(OR: 3.76; 95% CI [1.71-8.21]; OR: 1.84; 95% CI[1.06-3.18])。该模型预测PPI需要量的曲线下面积为0.73 (95% CI[0.67-0.79]),敏感性为0.74 (95% CI[0.47-0.93]),特异性为0.65 (95% CI[0.40-0.87])。结论建立了一种预测TAVR后PPI风险的预测模型,该模型结合了主动脉根部相关传导特异性的综合解剖测量和临床参数的电测量。该模型要求前瞻性应用程序了解其在现实世界中的性能。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Structural Heart
Medicine-Cardiology and Cardiovascular Medicine
CiteScore
1.60
自引率
0.00%
发文量
81
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


