Synergistic coupling of NiLa2O2(CO3) with MnO2/GO for durable and efficient water splitting in an alkaline condition
IF 6.3
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
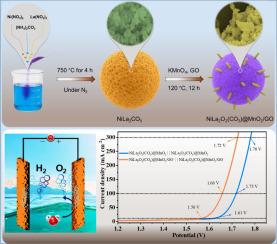
Water electrolysis represents a promising route for clean energy production, driving the development of innovative non-precious electrocatalysts to enhance efficiency and kinetic rates. In this work, the NiLa2O2(CO3)@MnO2/GO (NLOC@MnO2/GO) was prepared using a two-step approach involving pyrolysis and a hydrothermal process. The NLOC@MnO2/GO electrocatalysts showed a lower overpotential of oxygen evolution reaction (OER) for 190 mV and hydrogen evolution reaction (HER) for 78 mV at the current density of 10 mA cm-2. Moreover, it exhibited excellent electrocatalytic stability over 55 h at a high current density of 50 and -100 mA cm-2. The NLOC@MnO2/GO served as both electrode (cathode and anode) in a two-electrode water splitting device, delivering 10, 100, and 300 mA cm-2 at 1.56, 1.66, and 1.72 V, respectively. The exceptional activity arises from the intertwined architecture: Ni provides tunable electronic states, MnO2 introduces multi-electron redox centers, and GO scaffolds facilitate rapid ion/electron transport while preserving structural integrity. This study advances bifunctional electrocatalyst design and rare-earth engineering, integrating electronic modulation, active-site density, and mass transport in a single platform, while laying the foundation for future multi-functional, earth-abundant hybrid catalysts in clean energy applications.
NiLa2O2(CO3)与MnO2/GO的协同耦合在碱性条件下实现持久高效的水分解
水电解代表了清洁能源生产的一个有前途的途径,推动了创新的非贵重电催化剂的发展,以提高效率和动力学速率。本文采用热解和水热两步法制备了NiLa2O2(CO3)@MnO2/GO (NLOC@MnO2/GO)。在10 mA cm-2电流密度下,NLOC@MnO2/GO电催化剂的析氧反应过电位(OER)为190 mV,析氢反应过电位(HER)为78 mV。此外,在50和-100 mA cm-2的高电流密度下,它在55小时内表现出优异的电催化稳定性。NLOC@MnO2/GO在双电极水分解装置中同时充当电极(阴极和阳极),分别在1.56、1.66和1.72 V下输出10、100和300 mA cm-2。这种特殊的活性源于相互交织的结构:Ni提供了可调的电子态,MnO2引入了多电子氧化还原中心,GO支架促进了离子/电子的快速传递,同时保持了结构的完整性。该研究推进了双功能电催化剂设计和稀土工程,将电子调制、活性位点密度和质量输运集成在一个平台上,同时为未来多功能、地球丰富的混合催化剂在清洁能源中的应用奠定了基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Surfaces and Interfaces
Chemistry-General Chemistry
CiteScore
8.50
自引率
6.50%
发文量
753
审稿时长
35 days
期刊介绍:
The aim of the journal is to provide a respectful outlet for ''sound science'' papers in all research areas on surfaces and interfaces. We define sound science papers as papers that describe new and well-executed research, but that do not necessarily provide brand new insights or are merely a description of research results.
Surfaces and Interfaces publishes research papers in all fields of surface science which may not always find the right home on first submission to our Elsevier sister journals (Applied Surface, Surface and Coatings Technology, Thin Solid Films)
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


