Synthesis, in silico studies and in vitro evaluation of benzimidazole and benzotriazole derivatives as efflux pump inhibitors
IF 3.4
4区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
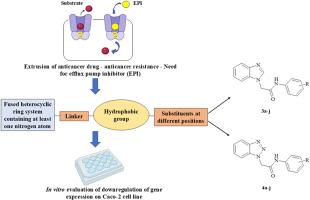
Multidrug resistance (MDR), primarily mediated by overexpression of ATP-binding cassette (ABC) transporters such as breast cancer resistance protein (BCRP) and P-glycoprotein (P-gp), pose a significant barrier to effective cancer chemotherapy. In this study, a series of benzimidazole (3a-3j) and benzotriazole (4a-4j) derivatives were rationally designed, synthesized, and characterised, aiming to develop efflux pump inhibitors with minimal intrinsic cytotoxicity. In silico docking studies against BCRP and P-gp revealed favourable binding profiles, with several compounds displaying key interactions with transporter residues. AutoQSAR-based predictive modelling further supported their potential, with disubstituted analogues emerging as promising candidates due to their superior predicted activity. Selected compounds were evaluated in vitro using MTT cytotoxicity assays and efflux inhibition studies in differentiated Caco-2 cells. Compounds 3c and 4c preferentially inhibited P-gp, while 3g showed higher BCRP inhibition, with compound 3c exhibiting dual activity and a favourable safety profile. Collectively, these findings suggest that the benzimidazole and benzotriazole scaffolds offer a viable strategy for the development of selective efflux pump modulators to combat MDR in cancer.
苯并咪唑和苯并三唑衍生物作为外排泵抑制剂的合成、硅研究和体外评价
多药耐药(MDR)主要由atp结合盒(ABC)转运体如乳腺癌耐药蛋白(BCRP)和p -糖蛋白(P-gp)的过表达介导,对有效的癌症化疗构成重要障碍。本研究对一系列苯并咪唑(3a-3j)和苯并三唑(4a-4j)衍生物进行了合理的设计、合成和表征,旨在开发具有最小内在细胞毒性的外排泵抑制剂。针对BCRP和P-gp的硅对接研究揭示了良好的结合谱,其中一些化合物与转运体残基表现出关键的相互作用。基于autoqsar的预测模型进一步支持了它们的潜力,双取代类似物因其优越的预测活性而成为有希望的候选物。选择的化合物通过MTT细胞毒性试验和分化Caco-2细胞的外排抑制研究进行体外评估。化合物3c和4c优先抑制P-gp,而3g具有更高的BCRP抑制作用,化合物3c具有双重活性和良好的安全性。总的来说,这些发现表明,苯并咪唑和苯并三唑支架为开发选择性外排泵调节剂来对抗癌症中的耐多药耐药提供了一种可行的策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
3.50
自引率
7.70%
发文量
492
审稿时长
3-8 weeks
期刊介绍:
The Journal of the Indian Chemical Society publishes original, fundamental, theorical, experimental research work of highest quality in all areas of chemistry, biochemistry, medicinal chemistry, electrochemistry, agrochemistry, chemical engineering and technology, food chemistry, environmental chemistry, etc.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


