Zinc vanadate-based nanocomposites: Structural, optical and photocatalytic studies
IF 3.4
4区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
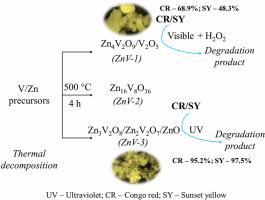
The preparation of zinc vanadate (ZnV)-based nanocomposites for their photocatalytic application has gained much interest. Herein, ZnV-based nanocomposites (ZnV samples) were synthesised by a thermal decomposition method. The variation in the structural components was investigated by changing the mole concentration of ammonium metavanadate/zinc acetate salts. The as-synthesised ZnV samples were characterized by X-ray diffraction (XRD), Raman and Fourier transform infrared (FTIR) spectroscopic techniques. The oxidation state of vanadium (V), zinc (Zn) and oxygen (O) was confirmed by X-ray photoelectron spectroscopic (XPS) analysis. By using electron microscopic analysis, the morphological features and particle size of ZnV samples were determined, while surface characteristics were analyzed by Brunauer-Emmett-Teller (BET) method. The ultraviolet (UV)/visible light absorption features and band-gap of the ZnV samples were determined via UV–visible spectroscopic analysis. The efficiency of ZnV samples in the photocatalytic degradation of Congo red and Sunset yellow dyes were determined under UV and visible light (with hydrogen peroxide, H2O2) irradiation separately. ZnV-3 sample (Zn3V2O7/Zn2V2O7/ZnO) is found to be active under UV and it showed better photocatalytic efficiency of 95.2 % (120 min) and 97.5 % (90 min) for Congo red and Sunset yellow degradation reactions, respectively.
钒酸锌基纳米复合材料:结构、光学和光催化研究
钒酸锌(ZnV)基纳米复合材料的制备及其光催化应用已引起人们的广泛关注。本文采用热分解法合成了ZnV基纳米复合材料(ZnV样品)。通过改变偏氰酸铵/乙酸锌盐的摩尔浓度,研究了结构组分的变化。采用x射线衍射(XRD)、拉曼光谱(Raman)和傅里叶变换红外光谱(FTIR)对合成的ZnV样品进行了表征。用x射线光电子能谱(XPS)分析证实了钒(V)、锌(Zn)和氧(O)的氧化态。通过电镜分析确定了ZnV样品的形貌特征和粒度,并用BET法分析了ZnV样品的表面特征。通过紫外可见光谱分析测定了ZnV样品的紫外/可见光吸收特性和带隙。分别测定了ZnV样品在紫外光和可见光(过氧化氢、H2O2)照射下光催化降解刚果红和日落黄染料的效率。Zn3V2O7/Zn2V2O7/ZnO对刚果红和日落黄的光催化效率分别为95.2% (120 min)和97.5% (90 min)。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
3.50
自引率
7.70%
发文量
492
审稿时长
3-8 weeks
期刊介绍:
The Journal of the Indian Chemical Society publishes original, fundamental, theorical, experimental research work of highest quality in all areas of chemistry, biochemistry, medicinal chemistry, electrochemistry, agrochemistry, chemical engineering and technology, food chemistry, environmental chemistry, etc.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


