Vitamin C–functionalized copper nanozymes for treating drug-resistant intracellular infections and hyperinflammation
IF 10.2
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
Bacterial infections, particularly drug-resistant intracellular infections, remain a major clinical challenge due to antibiotic resistance and immune evasion. Here, we report a vitamin C–functionalized copper-based nanozymes (CVs) as multifunctional therapeutic agents capable of simultaneously eradicating intracellular bacteria and mitigating hyperinflammation. Benefiting from excellent biocompatibility and acid-responsive activity, CVs exhibit potent bactericidal, reactive oxygen species (ROS)-scavenging, and immunomodulatory functions. Mechanistically, CVs enter bacterial cytoplasm via copper transporters, induce intracellular copper overload, disrupt the tricarboxylic acid cycle, and promote lipid peroxides accumulation, thereby triggering a cuproptosis-like bacterial death pathway. Moreover, CVs mimic superoxide dismutase activity to efficiently eliminate excessive ROS, and regulate immune responses by suppressing macrophage polarization toward the pro-inflammatory M1 phenotype while reducing pro-inflammatory cytokine production. These combined effects attenuate inflammation and promote tissue repair within infection-associated microenvironments. In a methicillin-resistant Staphylococcus aureus (MRSA)-induced peritonitis mouse model, CVs achieved robust intracellular bacterial clearance and inflammation resolution, underscoring their potential as next-generation copper-based nanomaterials for treating refractory intracellular bacterial infections and hyperinflammation-related disorders.
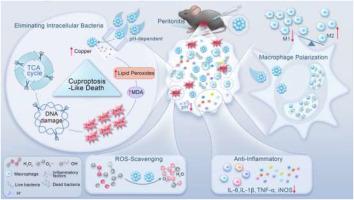
维生素c功能化铜纳米酶治疗耐药细胞内感染和过度炎症
细菌感染,特别是耐药细胞内感染,由于抗生素耐药性和免疫逃避,仍然是一个主要的临床挑战。在这里,我们报道了一种维生素c功能化的铜基纳米酶(CVs)作为多功能治疗剂,能够同时根除细胞内细菌和减轻过度炎症。得益于良好的生物相容性和酸反应活性,CVs具有强大的杀菌、活性氧(ROS)清除和免疫调节功能。机制上,CVs通过铜转运体进入细菌细胞质,诱导细胞内铜超载,破坏三羧酸循环,促进脂质过氧化物积累,从而触发铜中毒样细菌死亡途径。此外,CVs模拟超氧化物歧化酶活性,有效消除过多的ROS,并通过抑制巨噬细胞向促炎M1表型的极化,同时减少促炎细胞因子的产生来调节免疫反应。这些综合作用减轻炎症和促进组织修复感染相关的微环境。在耐甲氧西林金黄色葡萄球菌(MRSA)诱导的腹膜炎小鼠模型中,CVs实现了强大的细胞内细菌清除和炎症解决,强调了它们作为下一代铜基纳米材料治疗难治性细胞内细菌感染和高炎症相关疾病的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Materials Today Bio
Multiple-
CiteScore
8.30
自引率
4.90%
发文量
303
审稿时长
30 days
期刊介绍:
Materials Today Bio is a multidisciplinary journal that specializes in the intersection between biology and materials science, chemistry, physics, engineering, and medicine. It covers various aspects such as the design and assembly of new structures, their interaction with biological systems, functionalization, bioimaging, therapies, and diagnostics in healthcare. The journal aims to showcase the most significant advancements and discoveries in this field. As part of the Materials Today family, Materials Today Bio provides rigorous peer review, quick decision-making, and high visibility for authors. It is indexed in Scopus, PubMed Central, Emerging Sources, Citation Index (ESCI), and Directory of Open Access Journals (DOAJ).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


