Dual-metal-organic framework and gallic acid incorporated 3D-printed scaffolds: Revolutionizing refractory bone defect repair through immune-angiogenic-neurogenic synergy
IF 10.2
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
Steroid-associated osteonecrosis (SAON)-related bone defects are refractory and present significant therapeutic challenges due to dysregulated multiple cellular functions and disrupted multidimensional microenvironments. Despite progress in regulating immune responses and promoting vascularization for SAON-related bone defects, effective neural innervation strategies remain limited. Notably, immune response, angiogenesis, and neural innervation are interdependent processes that collectively regulate bone regeneration. Herein, we engineered a novel 3D-printed composite scaffold with highly interconnected porosity and multiple bioactivities by integrating magnesium-copper dual-metal-organic framework (MgCu-MOF74), gallic acid (GA) and polylactic acid (PLA). MgCu-MOF74 exhibits antioxidant capacity, controllable release of metal ions, and osteo-angiogenic properties. The composite scaffold demonstrated excellent mechanical properties and degradation characteristics well suited for bone regeneration. More importantly, the incorporation of GA and dual-ion synergy enabled the scaffold to achieve pronounced multicellular modulation by promoting macrophage polarization, inducing endothelial cell-mediated angiogenesis, stimulating Schwann cell morphological maturation, and enhancing the osteogenic differentiation of bone marrow-derived mesenchymal stem cells (BMSCs), while markedly increasing intercellular crosstalk to optimize the local multidimensional microenvironment. In vivo studies further confirmed that the scaffold effectively facilitates the repair of SAON-related bone defects by harnessing the synergistic interactions among the immune, angiogenic, and neurogenic microenvironments. This work provides an innovative strategy for treating refractory SAON - related bone defects, highlighting the potential of the developed scaffold in modulating diverse cell types and remodeling complex microenvironments.
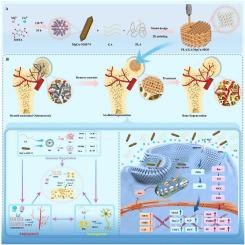
双金属有机框架和没食子酸结合3d打印支架:通过免疫-血管生成-神经生成协同作用彻底改变难治性骨缺损修复
类固醇相关性骨坏死(SAON)相关的骨缺损是难治性的,并且由于多种细胞功能失调和多维微环境破坏而呈现出重大的治疗挑战。尽管在调节免疫反应和促进saon相关骨缺损的血管化方面取得了进展,但有效的神经支配策略仍然有限。值得注意的是,免疫反应、血管生成和神经支配是相互依存的过程,共同调节骨再生。在此,我们通过整合镁铜双金属有机骨架(MgCu-MOF74)、没食子酸(GA)和聚乳酸(PLA),设计了一种具有高度互联孔隙度和多种生物活性的新型3d打印复合支架。MgCu-MOF74具有抗氧化能力、金属离子可控释放和成骨血管生成特性。复合支架具有良好的力学性能和降解特性,非常适合骨再生。更重要的是,GA的加入和双离子协同作用使支架能够通过促进巨噬细胞极化、诱导内皮细胞介导的血管生成、刺激雪旺细胞形态成熟、增强骨髓间充质干细胞(BMSCs)的成骨分化,同时显著增加细胞间的互扰,优化局部多维微环境,从而实现明显的多细胞调节。体内研究进一步证实,该支架通过利用免疫、血管生成和神经生成微环境之间的协同相互作用,有效促进了saon相关骨缺损的修复。这项工作为治疗难治性SAON相关骨缺损提供了一种创新策略,强调了开发的支架在调节多种细胞类型和重塑复杂微环境方面的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Materials Today Bio
Multiple-
CiteScore
8.30
自引率
4.90%
发文量
303
审稿时长
30 days
期刊介绍:
Materials Today Bio is a multidisciplinary journal that specializes in the intersection between biology and materials science, chemistry, physics, engineering, and medicine. It covers various aspects such as the design and assembly of new structures, their interaction with biological systems, functionalization, bioimaging, therapies, and diagnostics in healthcare. The journal aims to showcase the most significant advancements and discoveries in this field. As part of the Materials Today family, Materials Today Bio provides rigorous peer review, quick decision-making, and high visibility for authors. It is indexed in Scopus, PubMed Central, Emerging Sources, Citation Index (ESCI), and Directory of Open Access Journals (DOAJ).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


