Matrix-bound nanovesicles isolated from decellularized tumors as platforms for targeting parent tumor cells and tumor-associated stromal cells
IF 10.2
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
Matrix-bound nanovesicles (MBVs) are an emerging class of extracellular vesicles (EVs) that are integrated into the extracellular matrix (ECM). Tumor ECM-derived MBVs hold promise as platforms for targeted delivery of therapeutic agents to both parental tumor cells and surrounding stromal cells. In this study, a subcutaneous tumor model was established by implanting A549 human lung adenocarcinoma cells into immunodeficient mice. The mixed method was used to decellularize the tumor tissue, producing an ECM scaffold free of cellular components. Subsequently, MBVs were successfully isolated from the ECM of the decellularized tumors. Compared with tumor cell-derived liquid-phase EVs, acellular tumor MBVs were smaller in size and were demonstrated to transport proteins related to focal adhesion and protein binding. The in vitro binding affinity assays and cell culture experiments involving acellular tumor MBVs showed specific targeting affinity for ECM components, tumor cells, and tumor-associated stromal cells, including cancer-associated fibroblasts and tumor-associated macrophages. After loading of the drug doxorubicin, this platform selectively inhibited tumor cells and tumor-associated stromal cells both in vitro and in vivo. These results provide important insights for future research on the potential role of tumor ECM-derived MBVs in targeted cancer therapy and the modulation of premetastatic niches.
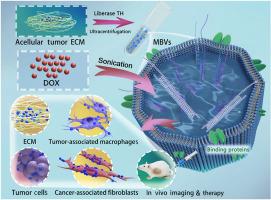
从去细胞化肿瘤中分离的基质结合纳米囊泡作为靶向亲本肿瘤细胞和肿瘤相关基质细胞的平台
基质结合纳米囊泡(mbv)是一类新兴的细胞外囊泡(ev),它们被整合到细胞外基质(ECM)中。肿瘤ecm衍生的mbv有望成为靶向递送治疗药物到亲代肿瘤细胞和周围基质细胞的平台。本研究通过将A549人肺腺癌细胞植入免疫缺陷小鼠,建立皮下肿瘤模型。采用混合方法对肿瘤组织进行脱细胞处理,制备无细胞成分的ECM支架。随后,从脱细胞肿瘤的ECM中成功分离出mbv。与肿瘤细胞衍生的液相ev相比,脱细胞肿瘤mbv体积更小,并被证明可运输与局灶黏附和蛋白质结合相关的蛋白质。非细胞肿瘤mbv的体外结合亲和力测定和细胞培养实验显示,其对ECM成分、肿瘤细胞和肿瘤相关基质细胞(包括癌症相关成纤维细胞和肿瘤相关巨噬细胞)具有特异性靶向亲和力。负载药物阿霉素后,该平台在体外和体内均选择性抑制肿瘤细胞和肿瘤相关基质细胞。这些结果为未来研究肿瘤ecm衍生的mbv在靶向癌症治疗和转移前生态位调节中的潜在作用提供了重要见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Materials Today Bio
Multiple-
CiteScore
8.30
自引率
4.90%
发文量
303
审稿时长
30 days
期刊介绍:
Materials Today Bio is a multidisciplinary journal that specializes in the intersection between biology and materials science, chemistry, physics, engineering, and medicine. It covers various aspects such as the design and assembly of new structures, their interaction with biological systems, functionalization, bioimaging, therapies, and diagnostics in healthcare. The journal aims to showcase the most significant advancements and discoveries in this field. As part of the Materials Today family, Materials Today Bio provides rigorous peer review, quick decision-making, and high visibility for authors. It is indexed in Scopus, PubMed Central, Emerging Sources, Citation Index (ESCI), and Directory of Open Access Journals (DOAJ).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


